[box]Price of Pregnancy: [/box]
While IVF may be tremendously gratifying for parents rearing healthy babies, a fraction of IVF-conceived children are afflicted by a host of issues. Developmental, cognitive, musculoskseletal, and even related maternal harms warrant attention as literal and figurative “costs” of IVF. Children born out of IVF tend to be preterm and/or small-for-gestational age (SGA) with respect to the parturient population4. After extensive matching on maternal characteristics (age, parity, ethnic origin, height, weight, smoking habits, obstetric/medical history), pregnancy outcome, delivery, neonatal outcome, pueriperium, and obstetric department in four Dutch university centers, authors Koudstaal et al discovered that gestational age at delivery was about 3 days shorter in IVF pregnancies. In addition, nearly two-thirds of the Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes data set was comprised of “preemie” babies, and SGA was present in half of those4.
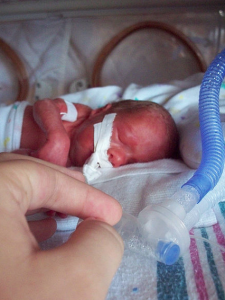 What is more, higher SGA proportions in the IVF group (16.2 as opposed to 7.9%) necessitated more intensive neonatal care4. Adverse Outcomes of IVF/ICSI Pregnancies Vary Depending on Aetiology of Infertility also utilized Danish national registries to compile preterm and normal delivery statistics. Judging outcomes of 225 IVF pregnancies against those of a 26,870 Finnish controls (naturally conceived live births), Langhoff-Roos et al confirmed that IVF significantly increased odds of having an “extremely preterm” delivery rather than a “very preterm” delivery5. Other studies have concluded that IVF pregnancies were associated with, “higher incidences of preterm birth, low birth weight, small for gestational age (SGA) infants, and NICU admissions”6.
What is more, higher SGA proportions in the IVF group (16.2 as opposed to 7.9%) necessitated more intensive neonatal care4. Adverse Outcomes of IVF/ICSI Pregnancies Vary Depending on Aetiology of Infertility also utilized Danish national registries to compile preterm and normal delivery statistics. Judging outcomes of 225 IVF pregnancies against those of a 26,870 Finnish controls (naturally conceived live births), Langhoff-Roos et al confirmed that IVF significantly increased odds of having an “extremely preterm” delivery rather than a “very preterm” delivery5. Other studies have concluded that IVF pregnancies were associated with, “higher incidences of preterm birth, low birth weight, small for gestational age (SGA) infants, and NICU admissions”6.
Marlow et al further contends that births after less than 26 weeks of gestation have a higher prevalence of neurological and developmental disabilities within the first two years of life. In the UK and Ireland, children born in 1995 at less than or equal to 25 weeks of gestation were evaluated for cognitive impairments at 30 months of age7. “Disability” was characterized as: severe, moderate, or mild, and classmates were matched for age and sex as controls. Each subject was scored by seven developmental pediatricians and eight psychologists on the Kaufman Assessment Battery for Children scale (K-ABC). Mental Processing Composite Scores were significantly lower in preterm cohorts on the order of 10 and 9 points for boys and girls respectively, whereas all of the control infants boasted similar scores across four scales of the K-ABC7. M
Marlow’s study elucidates the very real propensity of IVF births to manifest in cognitive, neuromotor, vision, hearing, and overall disability. Twenty percent of extremely preterm babies later revealed signs of spastic and dyskinetic cerebral palsy, and 15% displayed spastic diplegia7. Statistics highlighting even greater costs are that 21% of IVF children exhibiting some form of cognitive impairment, 12% with cerebral palsy, and of 86% still demonstrating severe retardation at age six4. Fifteen of those children were non-walkers, fifteen had moderate motor disabilities, four were hemiplegic, and nine were quadriplegic7. Observations in Marlow, Langhoff-Roos, and Koudstaal’s studies illustrate that greater cognitive impairment and poorer educational achievement were repeatedly noted in school-age kids of extremely low birth weight versus those born at full gestational term, raising concerns of costs beyond payment simply for IVF procedures.
Besides neo-natal issues, maternal vaginal blood loss, placenta praevia, and Caesarean sections stem from preterm pregnancies4. Preterm births are also connected to spontaneous abortions or stillbirths, history of two or more induced abortions, young maternal age, low pre-pregnancy weight, nulliparity, previous preterm birth, intrauterine exposure to DES, incompetent cervix, uterus anomaly, pylelonephritis, and low weekly weight gain—all of which demand rigorous medical, and hence economical, attention4.
[box]Modeling the IVF Market: [/box]
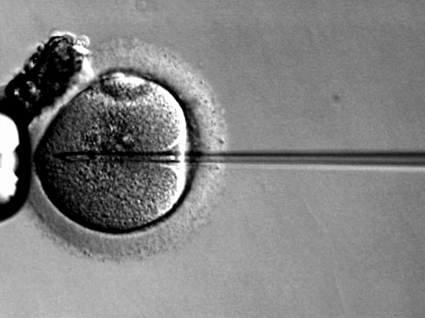 Variances between IVF and control pregnancies yield maladies feeding skyrocketing healthcare expenditures4. As of late, there have been several cost-effectiveness comparisons across IVF and other infertility solutions available. Previous IVF decision-making models have not been realistic. Pragmatic models must account for cancelled cycles, decreases in pregnancy rates of subsequent cycles, frozen embryo transfers, and dropout. Pashayan et al sought to model the cost-effectiveness of primary offer IVF as opposed to IVF in partners after IUI attempts fell short. Intrauterine Insemination (IUI) and In-Vitro Fertilization (IVF) have been deemed first-line treatments for couples ailing from unexplained or male subfertility3. IUI, sadly, is seldom successful on its own. Ensuing IVF treatment tacks on to already lofty prices of infertility. UK’s 1995-1999 Human Fertilization and Embryology Authority (HFEA) population-based register served as the standard for average live birth rates of IVF pregnancies. NICE, the UK National Institute for Clinical Excellence, advocates for six cycles of Unstimulated Intrauterine Insemination (U-IUI) for unexplained or male subfertility3. Consistent with NICE’s recommendation, Pashayan’s model mimics the clinical experience of 100 hypothetical couples randomized to either six rounds of: U-IUI + 1 round of IVF, S-IUI (Stimulated IUI) + 1 round of IVF, or simply 1 round of IVF.
Variances between IVF and control pregnancies yield maladies feeding skyrocketing healthcare expenditures4. As of late, there have been several cost-effectiveness comparisons across IVF and other infertility solutions available. Previous IVF decision-making models have not been realistic. Pragmatic models must account for cancelled cycles, decreases in pregnancy rates of subsequent cycles, frozen embryo transfers, and dropout. Pashayan et al sought to model the cost-effectiveness of primary offer IVF as opposed to IVF in partners after IUI attempts fell short. Intrauterine Insemination (IUI) and In-Vitro Fertilization (IVF) have been deemed first-line treatments for couples ailing from unexplained or male subfertility3. IUI, sadly, is seldom successful on its own. Ensuing IVF treatment tacks on to already lofty prices of infertility. UK’s 1995-1999 Human Fertilization and Embryology Authority (HFEA) population-based register served as the standard for average live birth rates of IVF pregnancies. NICE, the UK National Institute for Clinical Excellence, advocates for six cycles of Unstimulated Intrauterine Insemination (U-IUI) for unexplained or male subfertility3. Consistent with NICE’s recommendation, Pashayan’s model mimics the clinical experience of 100 hypothetical couples randomized to either six rounds of: U-IUI + 1 round of IVF, S-IUI (Stimulated IUI) + 1 round of IVF, or simply 1 round of IVF.
Cost was interpreted as the sum of IUI and IVF health services, with an incremental cost-effectiveness ratio represented by: [((cost of “IUI + IVF” arrangements) – (cost of IVF))/(difference in number of live births produced)]. Six cycles of U-IUI and one cycle of IVF totaled £495,9003. ICER for a primary round of IVF versus U-IUI and subsequent IVF was cited as £18,000 to £14,200 per live birth, and rose with each auxiliary IVF cycle. In the same vein, cost of an S-IUI + IVF combination was circa £369,000, but could reach up to a maximum of £759,800. ICER of regular IVF weighed against S-IUI + IVF ranged from £15,000 to £13,000 for each newborn3. To put costs in a more societal perspective, six cycles of IUI followed by one IVF cycle amplifies the total fertility expenditure of the 100 modeled couples by a minimum of £174,200 (3). More unsettling, this deficit bars 54 (out of the supposed 100) couples from acquiring IVF treatment, and at least 14 of those parents would have achieved live births3. Ultimately, it is more cost-effective for couples with male or unexplained subfertility to solely undergo IVF in preference to pursuing IUI. Even when only S-IUI is employed in lieu of U-IUI, costs up to £438,000 is prevented—funds sufficient to cover 136 IVF cycles, facilitating no less than 35 live births. Opportunity costs of IUI-use induce unwanted economic and psychological wastefulness3.
Nelson et al is yet another UK-based research group jumping on the IVF prediction model bandwagon. Past studies have been tainted by inadequate sample size and lack of external validation. Accordingly, Nelson et al developed a prototype “feasible with moderate discrimination and excellent calibration” (1). Utilizing the Human Fertilisation and Embryology Authority database, predictors of live birth (the universal unit of success in the realm of IVF research) were examined both in IVF and spontaneous pregnancies taking place between 2003 and 20079. This model uniquely acknowledged effects of donor oocytes, ICSI cycle frequency, previous spontaneous or IVF-related births, and fetal loss to more accurately predict healthy pregnancies. As a result, Nelson et al learned that odds of one live birth was 23.4 per 100 IVF cycles.
Another such model is the Markov model of Fiddelers et al. This model centered around two characteristics: a maximum of three cycles, and choice of “similar” or “constructive” transfer policies. “Similar” referred to one of the following recipes: (1 eSET + 2 STP), (1 eSET + 2 DET), (1 eSET + 1 STP + 1 DET), or (1 STP + 2 DET). Conversely, “constructive” transfer policy consisted of either three eSET, three STP, or three DET cycles8. Total fertility failure is defined as: no transfer, pregnancy with one or more children, complications such as miscarriage and stillbirth, or no pregnancy. Moreover, the Markov model delineates stopping IVF as: 2 cycles cancelled in a row, personal-initiated dropout, a maximum of three cycles (all variations: eSET, DET, or STP), or a live birth.
To generate this model, costs were calculated two weeks prior to randomization for each couple, and then once more six weeks thereafter. Overall, Fiddelers et al found that hormonal stimulation, laboratory fees, embryonic transfer, and maternal medical attention encompassed a majority of IVF expenses8. Second-order Monte Carlo Simulations communicated poor trajectories of cost-effectiveness for intermixture trials of the three ART selections. Contrastingly, three cycles of eSET, DET, or STP alone were highly cost-effective8. Among solo rounds of eSET, DET, and STP, cost-effectiveness thresholds were conveyed by cost per live birth. Three eSET cycles required an incremental cost increase of £7,350 for one auxiliary live birth. Alternatively, three rounds of STP call for £7350 – £15,250 cost increases for one live birth8. Lastly, while DET is the most efficacious of the techniques, it is also the priciest. Three DET cycles necessitate over £15,250 per live birth8.
[box]Cost of Contentment: [/box]
Although Cost-effectiveness of primary offer of IVF vs. primary offer of IUI followed by IVF (for IUI failures) in couples with unexplained or mild male factor subfertility, Cost-effectiveness of seven IVF strategies: results of a Markov decision-analytic model, and Prediction of live birth and extremes of response in stimulated cycles implications for individualization of therapy are novel in their field, policymakers must bear in mind that these models are limited by their inability to be extrapolated to the mainstream3. Technically, substantially larger cohorts in randomized, controlled trials accomplish this goal. Unfortunately, ethical review boards keep studies of this nature on tight leashes. In turn, obtaining sizable, homogeneous cohorts is practically impossible. IUI data, for instance, is heterogeneous in regard to subject demographics, ovarian stimulation regimes, inseminations per cycle, insemination timing, and methods of sperm preparation3. Omission of diagnostic, incident/follow-up appointment, and counseling fees (not to mention psychological anguish) renders these models inadequate. For many reasons, these models are not all-inclusive representations of cost—impermissible when estimating costs of politically polarizable health policies.
[box]ART and ACA – Antagonistic?:[/box]
By and large, insurance plans in the US fail to cover infertility. Distressingly, mandates are not necessarily consigned to where insured individuals live or work. Instead, the location of policy issuance dictates the obligations by which insurance companies abide. Common institutions exempt from requirements in these states are small businesses, religious organizations, and self-insured companies11. In 2014, reduced savings on non-covered tax-deductible procedures will cause costs for assisted reproductive technologies, namely, IVF, to intensify. Only $2500 will be allocated to Flexible Spending Accounts. Though a cap has not been placed, employers will self-select contribution limits10. Meanwhile, the deductible medical expenses threshold will be augmented from 7.5 to 10% of Adjusted Gross Income (AGI)10.
Fifteen of the fifty states have infertility insurance regulations: Arkansas, California, Hawaii, Illinois, Louisiana, Maryland, Massachusetts, Montana, New Jersey, New York, Ohio, Rhode Island, Texas, and West Virginia10. Nonetheless, precisely what “coverage” entails fluctuates greatly from state to state. On December 16, 2011, consumers, states, employers and issuers were furnished with more timely information as they work towards establishing Affordable Insurance Exchanges and making decisions for 2014. The Department of Health and Human Services issued a “comprehensive, affordable and flexible” bulletin with policies that giving states more flexibility in ACA protocol11. ACA of the future ensures that health plans (individual and small group markets both inside and outside of the Affordable Insurance Exchanges), offer a comprehensive package of items and services, known as “Essential Health Benefits.” Hinging on scientific and economical exploration, IVF may or may not become part and parcel of the Essential Health Benefits.
Activists, relatives, politicians, and infertility-plagued couples alike are striving to instate a novel federal bill to enhance IVF’s chances of attaining Essential Health Benefit status. “The Family Act” aims to bestow tax credits to couples struggling to surmount the overwhelming out-of-pocket costs of infertility treatment. Presently, families who adopt are tendered similar tax credits. Infertile couples are now demanding the same financial assistance to start their own families. Family Act Legislation would provide a maximum lifetime tax credit of $13,360 to families with an adjusted gross income of less than $182,500, and yearn for children. To utilize IVF, taxpayers must have been diagnosed as infertile by a licensed physician with an indicated course of treatment as IVF12. In November 2011, espousal for The Family Act grew leaps and bounds when Rep. John Lewis (D-GA) announced the legislation in the U.S. House of Representatives11. “For too many of those people, the cost of treatment will silence their dreams of having children forever. That’s not right, fair or just. I believe that access to decent health care should be a fundamental right, not a privilege,” stated Rep. Lewis in the hopes that The Family Act of 2011 will be instated12. Twenty-one members of the House co-sponsor the Family Act. A preponderance of Representatives aspiring for Family Act passage is Left-Wing-affiliated12.
Introduced to the Senate in May of 2011 by Kirsten Gillibrand, (D-NY), Daniel Akaka, HI, Daniel Inouye, HI, and Mary Landrieu, LA agreed to co-sponsor: all of whom hail from the Democratic Party as well12. Because The Family Act would also apply to treatments to preserve fertility for cancer patients, such as egg-freezing, big-name organizations, namely The American Cancer Society, American Congress for Obstetricians and Gynecologists, American Fertility Association, American Society for Reproductive Medicine, Leukemia and Lymphoma Society, Livestrong, National Hispanic Medical Association, RESOLVE, and the United States Hispanic Chamber of Commerce now lobby for Family Act efforts, too11. Progress of The Family Act of 2011, S 965/HR 3522, corresponds to 48% Senate support, with 4,830 alerts taken (with a goal of 10,000)12. Whether or not this bill is enacted into law, its potential incorporation into the Affordable Care Act remains to be seen.
[box]IVF for the Win:[/box]
An upsurge in IVF demand, and the snowballing costs of neonatal services aforementioned due to influx of IVF-related premature babies or multiple births has obviously sparked fear in the healthcare sphere1. It is inevitable that the rising tide of consumer demand of IVF as treatment options for a legitimate disease, will catalyze much needed change11. Infertility has a highly heterogeneous etiology, warranting supplementary research. External validity of maternal and natal studies and economical models must be bettered. Clinical efficacy of infertility treatments brings about a mass of unanswered questions: is there availability of PGD? Can healthcare incorporate infertility screening? Can severity of infertility be assessed via genotyping and preclude individuals from coverage? What will be the protocol for covering family counseling and planning? Health policy opens a whole new can of worms. If Essential Health Benefits sanctions treatment for infertility, what would be the immediate effects? Will adverse enrollee selection ensue? Fundamentally, IVF in US Health Policy is dependent on the value our nation should place on IVF “success”—one live birth.
References:
1. “The Oldest and Largest Infertility (INCIID) Adoption. Parenting, Pregnancy Online Community.” A History of IVF. The Oldest and Largest Infertility (INCIID) Adoption. Parenting, Pregnancy Online Community), 2004. Web. 20 May 2012. <http://www.inciid.org/printpage.php?cat=ivf>.
2. “The Cost-Effectiveness of IVF.” IVF News: The Cost-Effectiveness of IVF. American Society for Reproductive Medicine, 17 Oct. 2007. Web. 14 May 2012. <http://www.ivf.net/ivf/the-cost-effectiveness-of-ivf-o3027.html>.
3. Pashayan, N, Lyratzopoulos, G, Mathur, R. Cost-effectiveness of primary offer of IVF vs. primary offer of IUI followed by IVF (for IUI failures) in couples with unexplained or mild male factor subfertility. BMC Health Services Research, vol. 6, article 80, pp. 1–11, 2006.
4. Koudstaal, J., Braat, D.D.M, Bruinse, H.W., Naaktgeboren, N., Vermeiden, J.P.W., and Visser, G.H.A. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum Reprod (2006) 21(6): 1508-1513.
5. Langhoff-Roos J, Kesmodel U, Jacobsson B, et al. Spontaneous preterm delivery in primiparous women at low risk in Denmark: population-based study. BMJ 332(7547):937–9. 2006.
6. Kuivasaari-Pirinen P, Raatikainen K, Hippeläinen M, Heinonen S. Adverse Outcomes of IVF/ICSI Pregnancies Vary Depending on Aetiology of Infertility. ISRN Obstet Gynecol. 2012;2012:451915. Epub 2012 Apr 9.
7. Marlow, N., Wolke, D., Bracewell, M.A., & Samara, M., for the EPICure Study Group (2005). Neurologic and developmental disability at six years of age after extremely preterm birth. New England Journal of Medicine, 352, 9–19
8. Fiddelers AA, Dirksen CD, Dumoulin JC, van Montfoort AP, Land JA, Janssen JM, Evers JL, Severens JL: Cost-effectiveness of seven IVF strategies: results of a Markov decision-analytic model. Hum Reprod 2009, 24(7):1648-1655.
9. Nelson SM, Yates RW, Fleming R. Prediction of live birth and extremes of response in stimulated cycles implications for individualization of therapy. Hum Reprod 2007;22:2414–21.
10. “State Laws Related To Insurance Coverage for Infertility Treatment.” Insurance Coverage for Infertility Laws. National Conference of State Legislatures, Mar. 2012. Web. 15 May 2012. <http://www.ncsl.org/issues-research/health/insurance-coverage-for-infertility-laws.aspx>.
11. “The Affordable Care Act and Infertility.” The Affordable Care Act and Infertility RESOLVE: The National Infertility Association. RESOLVE: The National Infertility Association, 31 Jan. 2012. Web. 15 May 2012. <http://www.resolve.org/get-involved/the-affordable-ca
12. “Family Act of 2011 Takes a Positive Step Forward.” Family Act of 2011 Takes a Positive Step Forward. Fertilityauthority.com, Apr. 2011. Web. 20 May 2012. <http://www.fertilityauthority.com/articles/family-act-2011-takes-positive-step-forward>.
