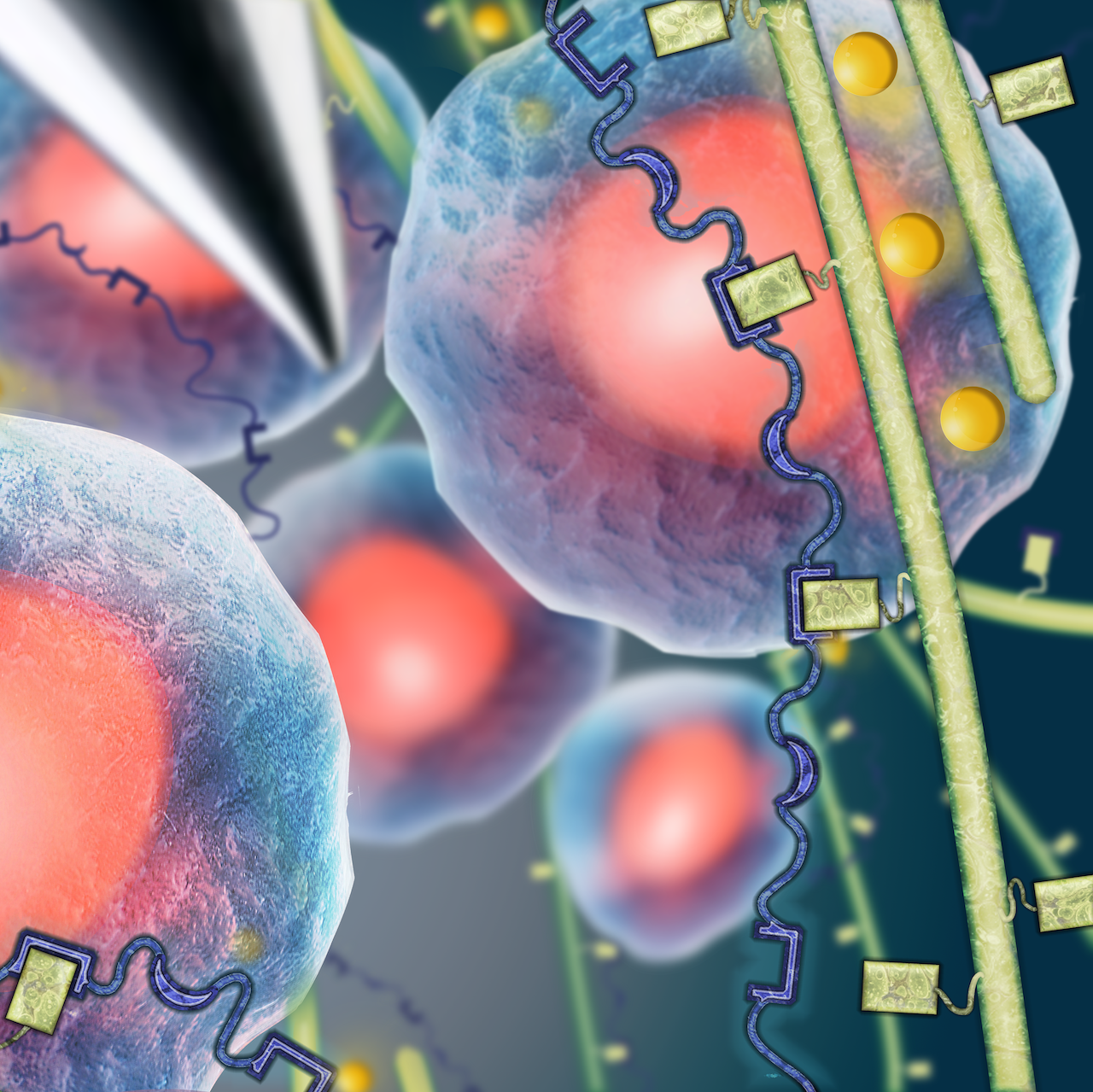
Cell transplantation has great potential for treating a wide variety of human diseases and injuries; however, due to a local inflammatory microenvironment and mechanical damage during the injection procedure, cell viability following simple injection protocols remains low. We are developing functional cell delivery materials to protect cells from mechanical stress during injection, localize them to the transplantation site, and direct their organization and differentiation in vivo. Our engineered materials are built from two classes of engineered proteins that create physically crosslinked hydrogels when mixed under constant physiological conditions. These mixing-induced three-component hydrogels (MITCH) allow cytocompatible 3D cell encapsulation and are shear-thinning and self-healing, making them ideal injectable vehicles for delivering encapsulated cells to a therapy site.
We are designing a new family of biomaterials that are made entirely of engineered proteins. By carefully selecting the primary amino acid sequence of our engineered proteins, we can create biomaterials with independently tunable biochemical and biomechanical properties that mimic many of the essential properties of natural tissues including elasticity, proteolytic remodeling, and cell binding and signaling. An essential component of these engineered protein-based materials are elastin-like peptide sequences that provide excellent mechanical resilience. These elastin-like biomaterials are being investigated for use both as ex vivo tissue mimics to study the fundamentals of cell-matrix interactions and as in vivo tissue mimics for regenerative medicine applications. Current systems under study include neuronal, cardiac, vascular, and bone tissues amongst others.
Recent advances in research and medicine have identified novel means to control human stem cell differentiation, maintenance, and proliferation in vitro. However, interactions between stem cells and their microenvironment represent a pressing area for investigation due to several limitations: 1) inadequately preserved 3D tissue architecture, 2) poorly-defined physiological parameters for in vitro culture, and 3) narrow understanding of cell-cell interactions during co-culture of stem cells with physiologically-appropriate adjacent cell types. To address these limitations, 3D organoid culture of patient-derived tissue samples or induced pluripotent stem cells (iPSCs) has emerged as a more biomimetic platform for modeling the interaction of human stem cells with their extracellular microenvironment. We hypothesize that the use of customizable, engineered protein scaffolds that mimic biochemical and biomechanical features of the native stem cell niche will provide unique insight into biological mechanisms of these stem cell-microenvironment interactions. To test our hypothesis and further understand these interactions, we culture a range of human organoid samples in our engineered materials including: intestinal, brain, liver, and cancer organoids.
Three-dimensional (3D) bioprinting has emerged as a promising tool for patterning cells and extracellular matrix components to rapidly produce complex and functional tissue-like structures. However, bioprinting is currently limited by the narrow range of materials that can be used as bioinks. The requirements for a successful biomaterial ink are two-fold. To fabricate complex, cell-laden structures, biomaterial inks must possess both appropriate mechanics to be printable and appropriate biocompatibility to support living cells. To achieve biological function, the material and biochemical properties of the ink must be tunable to fit the cellular and structural needs of the intended tissue application. Unfortunately, many widely used bioinks do not currently allow for this flexibility. To address this need, we are designing new bioinks that 1) use cell-compatible crosslinking methods, 2) can create cohesive structures using multiple biopolymers and cell types, and 3) are biochemically and mechanically customizable. By expanding the number and tunability of materials that are available for 3D bioprinting, we aim to fabricate complex, multi-material and multi-cellular constructs that better mimic in vivo architectures.