 |
|
| Home | Barry Trost | Group | Publications | Pictures | Contact | Stanford Chemistry | Chemistry Links | Stanford University | |
|
 _____ _____ 
------ Principal Research Interests Our research program revolves around the theme of synthesis. There are two major activities - 1) developing the tools, i.e., the reactions and reagents, and 2) creating the proper network of reactions to make complex targets readily available from simple starting materials.
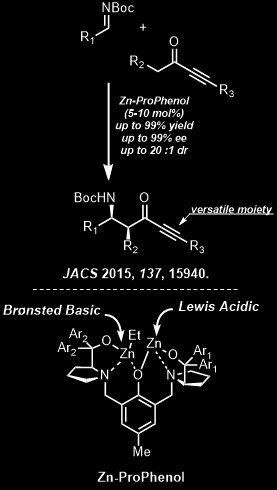
Synthetic efficiency raises the question of metal catalyzed cycloadditions to rings other than six membered. A general strategy for a "Diels-Alder" equivalent for formation of five, seven, nine, etc. membered carbo- and heterocyclic rings is evolving. An exciting new direction derives from the molecular gymnastics acetylenes undergo in the presence of transition metals. Cycloisomerization to virtually all types of ring sizes and systems with particularly versatile juxtaposition of functionality become special goals. While palladium catalysts represent a major part of our effort, new opportunities for selectivity complementary to that obtained with palladium complexes appear possible with nickel, chromium, molybdenum, ruthenium, iron, and tungsten complexes and bimetallic complexes composed of two different metals. Of prime concern are 1) exploration of unusual oxidation states of metals for new types of reactivity and 2) asymmetric templates that create enantiomerically pure stereogenic centers. Main group chemistry, especially involving silicon, tin, and sulfur, also offers many opportunities for new reaction design. For example, sulfur-containing substrates may function as either electrophiles or nucleophiles depending upon their chemical environment - a duality that allows them to be dubbed "chemical chameleons." Rational design of novel catalysts for an asymmetric direct aldol condensation is an exciting thrust. From these new synthetic tools evolve new synthetic strategies towards complex natural products. Targets include b-lactam antibiotics, ionophores, steroids and related compounds (e.g., Vitamin D metabolites), alkaloids, nucleosides, carbohydrates, and macrolide, terpenoid, and tetracyclic antitumor and antibiotic agents. |
| This website is designed and maintained by the Trost Group.
Please direct all questions or comments to schultz2 at stanford dot edu. Updated on March 30, 2017 |
|