Biomimetics and Bioconjugates
Part of my group is developing sequence and length-specific "polypeptoids" (poly-N-substituted glycines) or just “peptoids" for short, as biostable mimics of therapeutic peptides and proteins. Presently we are working to create peptoid mimics of lung surfactant proteins SP-B and SP-C, and also, peptoid analogues of antimicrobial peptides such as magainin or LL-37.
Sequence-Specific Peptoid Foldamers For Biomedical Applications
Our group has three main projects that focus on the development of sequence-specific poly-N-substituted glycines, or peptoids, for biomedical applications. Peptoids are non-natural, sequence-specific oligomers that are chemically synthesized in a manner similar to how polypeptides are made, and while they are based on the same backbone as peptides, the side chains of peptoids are appended to the amide nitrogen rather than to the a-carbon. Peptoids are protease resistant and are generally less expensive than peptides to synthesize, and in studies to date, appear to raise only a very low-level, non-specific immune response in vivo. The synthesis of a variety of different sequence-specific, biomimetic polypeptoids is done in our laboratory by the solid-phase "sub-monomer" synthesis method using ABI 433A automated peptide synthesizers; the peptoids are purified by reversed-phase HPLC and lyophilized to a powder, after which we can test them for their intended bioengineering application. Peptoids with bulky, a-chiral side chains have been shown to adopt stable folds in solution, including polyproline type I-like helices of controlled handedness, and a novel "threaded loop" structure.
Peptoid Mimics of Lung Surfactant Proteins SP-B and SP-C
Lung surfactant (LS) is a complex lipid-protein mixture that forms a thin film on alveolar surfaces of the lungs, reducing surface tension and enabling normal breathing. Premature infants born without functional LS suffer from neonatal respiratory distress syndrome (nRDS) and are routinely treated with animal-derived lung surfactants. While this treatment is efficacious, animal-derived surfactants are prohibitively expensive in many parts of the world, have a short shelf life, can vary in batch-to-batch effectiveness, and carry the risk of disease transmission, given the sourcing of this material directly from animal lungs. No synthetic, biomimetic surfactant is yet commercially available, primarily because none so far has proven to work as well in establishing normal respiratory function in neonates than animal surfactant. If a synthetic surfactant was available, there are many new clinical indications for its use, including (1) the treatment of adult/acute respiratory distress syndrome; (2) the prevention of ventilator-induced lung injury; and (3) the treatment of severe sepsis of the lung/bacterial pneumonia via delivery of a surfactant carrying antibiotics. Animal studies in universities and hospitals around the world already have proven the promise and efficacy of exogenous surfactant in treating these conditions; however, current bovine/porcine surfactants are too expensive to be used in adults due to larger required volumes and are also potentially immunogenic for children and adults (neonates have immature immune systems, so the risk of immune response with babies is very low).
To address the need for a low-cost, non-immunogenic surfactant replacement with a long shelf life, we are designing and characterizing, polypeptoid-based mimics of the two proteins in natural surfactant responsible for the surface tension-lowering properties of lung surfactant, Surfactant Protein B and C (SP-B and SP-C). We are supported by NIH/NHLBI for this work. Our goal is to develop a synthetic, biomimetic lung surfactant replacement that exhibits superior clinical efficacy to animal surfactants, while circumventing the many shortcomings of the currently available animal surfactants. We have made a large library of different polypeptoid-based mimics of SP-B (1-25) and SP-C, and in combination with lipids, some of these peptoid SP mimics appear highly promising in both in vitro and in vivo testing. The current focus of this work is the development of animal models, in collaboration with labs experienced in this area, with which to carry out thorough testing of our novel surfactant formulations. (see Brown NJ, Johansson J, Barron AE, "Biomimicry of surfactant protein C," Accounts of Chemical Research 2008, 41: 1409-1417; Brown NJ, Wu CW, Seurynck-Servoss SL, Barron AE, "Effects of hydrophobic helix length and side chain chemistry on biomimicry in peptoid analogues of SP-C," Biochemistry 2008, 47: 1808-1818.)
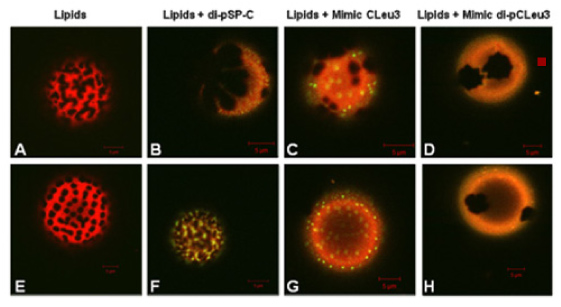
Below: Fluorescence microscopy images of peptoid-lipid and peptide-lipid Giant Unilamellar Vesicles (GUVs), where the different colors and phase morphology give us information about the extent of biomimicry we are observing in our peptoid mimics of Surfactant Proteins B and C. These images were obtained by former Ph.D. student Nate Brown, in collaboration with Dr. Jorge Bernadino de la Serna (MEMPHYS, Institut for Fysik og Kemi, Odense, Denmark). We also characterize our peptoid SP mimics using a Langmuir-Wilhelmy surface balance with fluorescence microscopic imaging of surface film morphology, and using a Pulsating Bubble Surfactometer that we have modified to allow visual tracking of bubble size and shape, calculating an accurate surface pressure vs. area hysteresis curve on the fl
Peptoid Mimics of Antimicrobial Peptides (AMPs)
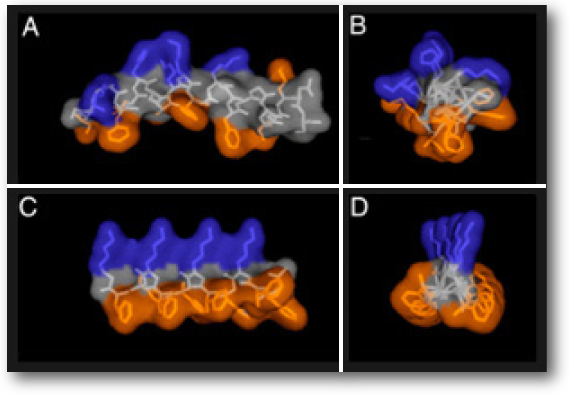
Figure: NMR structure of magainin-2 in DPPC micelles. (A and B) Parallel (A) and perpendicular (B) to its helical axis. (C and D) Similar views of a model structure of ampetoid 1. Because NLys is achiral, the structure of 1 is expected to be significantly dynamic in solution. Residues are color coded: cationic, blue; hydrophobic, orange; all others, gray. peptoid Mimic CLeu3 (C and G), and lipids with 10 wt% peptoid Mimic di-pCLeu3. Red corresponds to the fluorescently labeled lipid species and yellow to the labeled peptide and peptoids.
The development of resistance to conventional small-molecule antibiotics, which operate through receptor-mediated events, is an ever-increasing threat. Antibacterial peptides (AMPs) are naturally occurring components of innate immunity that function through a general, physical membrane permeabilization mechanism of action that is far less susceptible to the development of bacterial resistance. While the study of AMPs and their mechanism(s) of action have been an active area of research for almost 20 years, they have yet to see widespread clinical use; this is partly because the bioavailability of AMPs is limited because they are quickly destroyed by proteases. Peptidomimetic analogues of AMPs, based on a variety of chemical structures, have shown promise as more stable compounds that can kill bacteria by similar mechanisms.
With the support of the NIH/NIAID, the Barron lab is developing peptoid-based mimics of short, cationic, helical AMPs, which we have shown operate through a mechanism of action that is similar to natural AMPs. Protease-resistant peptoids are more bioavailable than peptides; our task is to ensure that they follow optimal metabolic/catabolic pathways of elimination from the body. In studies to date, we have shown that peptoid-based AMP mimics can exhibit relatively potent (low-µM) and extremely broad-spectrum activity against planktonic bacteria, and biofilms as well. This has been shown for both Gram-positive and Gram-negative, clinically relevant bacterial strains, including certain strains of bacteria that exhibit resistance to multiple classes of conventional antibiotics. We are working with other groups, which have clinical expertise and previously have developed AMPs as drugs, to test the ability of our antimicrobial peptoids ("ampetoids") to treat infections in vivo, in animal models. (see Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevitz D, Zuckermann RN, Barron AE, "Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides," PNAS 2008, 105: 2794-2799.; and Patch JA, Barron AE, "Helical peptoid mimics of magainin-2 amide," Journal of the American Chemical Society 2003, 125: 12092-12093.)