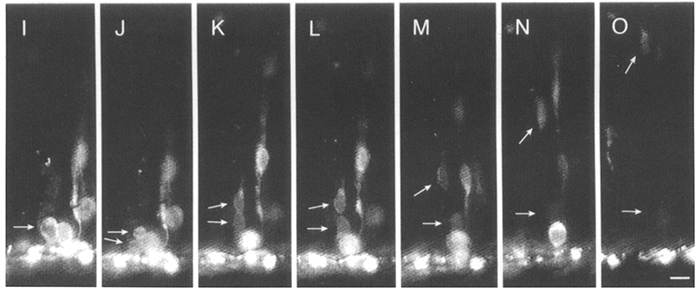
Neurons of the cerebral cortex are generated in the ventricular zone, a pseudostratified epithelium of progenitor cells that lines the lateral ventricles. Prior to the onset of neurogenesis, ventricular cells divide in a proliferative mode to expand the progenitor cell population. As neuronal production commences, cells begin to generate neurons, but they also produce more progenitors to replenish the precursor pool. As neurogenesis proceeds, more and more divisions give rise to neurons, until the rate of neuronal production peaks during the last third of neurogenesis. Finally, as neurogenesis comes to a close, progenitors probably divide in a terminal fashion, giving rise to neurons and depleting the precursor pool. Some time ago, we performed time-lapse imaging studies in cortical slices, which suggested that progenitor cells can divide asymmetrically during the period of neurogenesis, and that cleavage orientation of dividing precursors predicts the fate of the daughters (Chenn and McConnell, 1995). These studies suggested that vertically-oriented divisions generate two morphologically and behaviorally identical daughters, whereas horizonal divisions are asymmetric, generating one daughter that differentiates into a neuron and another that reenters the cell cycle. Finally, our data suggested that an asymmetric inheritance of Notch protein might contribute to the distinct fates of daughter cells in asymmetric divisions.
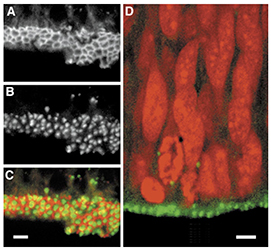
We also explored the role of apico-basal polarity in neurogenesis (Srinivasan et al., 2008). Apico-basal polarity plays an important role in regulating asymmetric cell divisions by neural progenitor cells in invertebrates, but the role of polarity in mammalian progenitor cells remains poorly understood. We have focused on the function of the PDZ domain protein MALS-3, which is localized to the apical domain of progenitors and is closely associated with the cell membrane. Analysis of mice lacking all three MALS genes revealed that MALS-3 is required for the normal apical localization of the polarity proteins PATJ and PALS1 but not for the formation or maintenance of adherens junctions. Loss of MALS altered early neurogenesis, since cells showed a decrease in BrdU incorporation at E11; they progressed more slowly through the cell cycle and showed increased cell cycle exit and differentiation into neurons. Interestingly, these effects were transient, since cycling progenitors recovered normal cell cycle properties a few days later. Gain-of-function experiments in which MALS-3 was targeted to the entire membrane resulted in a breakdown of both apico-basal polarity and adherens junctions, resulting in a disruption of the ventricular surface and the appearance of NPCs in the lateral ventricles. These results suggest that the maintenance of apico-basal polarity is required for normal neurogenesis.
Finally, we explored the roles of cilia and of small Rho family GTPases in regulating the behavior of progenitor cells in the ventricular and subventricular zones of the developing cortex.
References:
Wilson SL, Wilson JP, Wang C, Wang B, McConnell SK (2011) Primary cilia and Gli3 regulate cerebral cortical size. Dev. Neurobiol. doi:10.1002/dneu.20985.
Leone DP, Srinivasan K, Brakebusch C, McConnell SK (2010) The Rho GTPase Rac1 is required for proliferation of intermediate progenitors in the developing forebrain. Dev. Neurobiol. 70:659-678.
Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM, McConnell SK, Palmer TD (2009) A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Molec. Cell. Neurosci. 41:409-419.
Srinivasan S, Roosa J, Olsen O, Lee SH, Bredt DS, McConnell SK (2008) MALS-3 regulates polarity and early neurogenesis in the developing cerebral cortex. Development 135: 1781-1790.
Chenn A, McConnell SK (1995) Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82:631-641.



