We have explored the mechanisms that generate distinct neuronal phenotypes within the mammalian cerebral cortex, the layers of which contain neurons with strikingly similar morphologies and axonal connections. Because the "birthday" of a cortical neuron predicts its final position and connections, we can examine the developmental potential of these cells by transplanting them into different-aged brains, which might provide altered environmental cues for development. Our transplantation experiments have revealed that early progenitors, which normally produce deep layer neurons, are multipotent: these cells can directly produce upper layer neurons when transplanted into an older brain environment (McConnell and Kaznowski, 1991). Interestingly, the competence of progenitors to respond to fate-inducing cues changes over developmental time. The progenitors of layer 4 neurons retain the ability to differentiate into later-generated types when transplanted into older hosts, but have lost the ability to form layer 6 neurons if transplanted into younger brains (Desai and McConnell, 2000). The progenitors of layer 2/3 neurons are even more restricted; when transplanted into younger hosts, these cells generate only upper layer neurons, even if they divide again in the new environment (Frantz and McConnell, 1996).
Much of the in our lab has been geared toward identifying and understanding the molecular mechanisms that enable neurons to differentiate in a layer-specific manner. Some of our progress is summarized below.
Fezf2 is required for the normal development of subcortical projection neurons
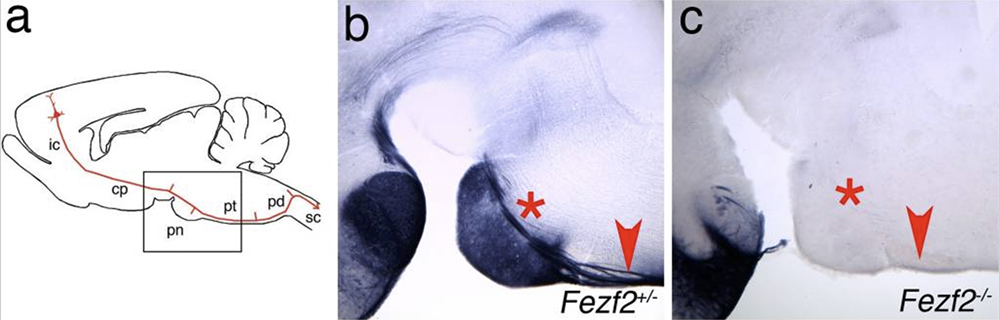
One prominent class of projection neurons in the cortex are the deep layer cells that extend long-distance axons to subcortical targets. Within the cortex, the zinc-finger transcription factors Fezf2 and Ctip2 are expressed by subcortically projecting neurons in layer 5 and in the corticothalamic projection neurons of layer 6. The axons of Fezf2-expressing neurons were visualized in genetic targeting experiments in which the Fezf2 open reading frame was replaced by a gene encoding the axonal marker placental alkaline phosphatase (PLAP). We found that genetic knockouts targeting the Fezf2 locus in mice results in striking alterations in the development of corticospinal motor neurons (CSMNs), in that their axons fail to extend into the corticospinal tract (CST) (Chen et al., 2005). We also observed defects in projections to other subcortical targets, including the midbrain and pons. Importantly, Ctip2 expression is lost in the cortices of Fezf2 mutant mice (but not in other brain regions), consistent with the suggestion that Fezf2 acts upstream of Ctip2 during cortical development. Experiments performed in knockout animals and in mouse chimeras suggest that Fezf2 regulates a fate switch: in its absence, many layer 5 and 6 neurons acquire the phenotypes of callosal projection neurons (Chen et al. 2008). These cells adopt the axonal projection patterns, physiological properties, and molecular marker expression patterns that are characteristic of callosal projection neurons. Conversely, the ectopic expression of Fezf2 in neurons that normally form corticocortical and callosal projections is sufficient to redirect their axons to subcortical targets.
Satb2 is required for the normal development of callosal projection neurons
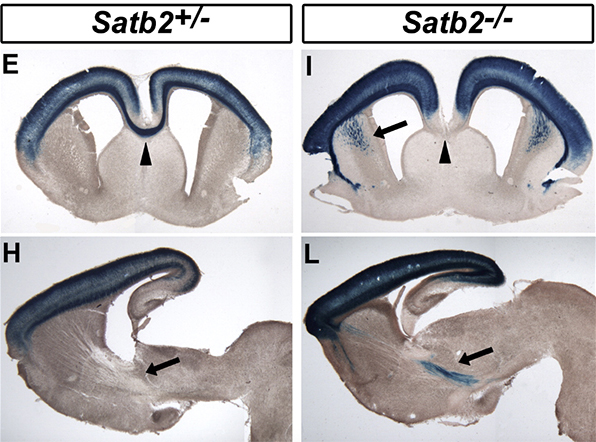
Neurons that extend axons across the corpus callosum to the opposite cerebral hemisphere comprise a subtype of neurons that form corticocortical connections. Callosal projection neurons are particularly prominent in the upper layers, although they are present in the deep layers as well. Our studies revealed that callosal projection neurons require the chromatin remodeling protein Satb2 for the formation of their normal projections, and that in the absence of Satb2, these cells extend axons toward subcortical targets (Alcamo et al., 2008). The axons of Satb2-expressing neurons were visualized in genetic targeting experiments in which the Satb2 open reading frame was replaced by a LacZ gene (Alcamo et al., 2008). In addition to adopting the axon trajectory of deep layer neurons, Satb2 mutants display alterations in the expression of several axon guidance molecules and a dramatic expansion of Ctip2 expression into the upper layers and within the deep layers, although the expression of Fezf2 was not obviously affected. These data suggest that Satb2 functions to repress the expression of Ctip2 in callosal projection neurons. Indeed, the repression of Ctip2 by Satb2 appears to be direct: Satb2 binds directly to matrix attachment regions (MARs) in the Ctip2 locus, where it recruits histone deacetylases and modifies chromatin configuration to assume a less activated state.
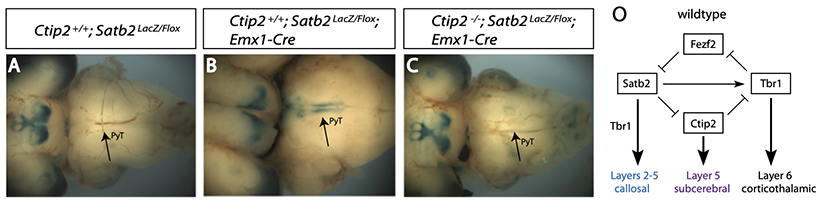
Subsequent work in our lab was directed toward understanding the genetic and biochemical interactions between these pathways, and to identifying and understanding the roles of other genes that specify or modify the fates of projection neurons in the developing cerebral cortex. By generating double mutants of Fezf2, Ctip2, and Satb2, we found that that cortical neurons deploy a complex genetic switch that uses mutual repression to produce subcortical or callosal projections. We discovered that Tbr1, EphA4, and Unc5H3 are critical downstream targets of Satb2 in callosal fate specification. This represents a unique role for Tbr1, implicated previously in specifying corticothalamic projections. We further showed that Tbr1 expression is dually regulated by Satb2 and Ctip2 in layers 2–5.
References:
Tsyporin J, Tastad D, Ma X, Nehme A, Finn T, Huebner L, Liu G, Gallardo D, Makhamreh A, Roberts JM, Katzman S, Sestan N, McConnell SK, Yang Z, Qiu S, Chen B (2021) Transcriptional repression by FEZF2 restricts alternative identities of cortical projection neurons. Cell Reports 35: 109269. doi.org/10.1016/j.celrep.2021.109269
Heavner WE, Ji S, Notwell JH, Dyer E, Tseng A, Yoo B, Birgmeier J, Bejerano G, McConnell SK (2020) Transcription factor expression defines subclasses of developing projection neurons highly similar to single-cell RNAseq subtypes. Proc. Natl. Acad. Sci. USA 117:25074-25084.
Leone DP, Panagiotakos G, Heavner WE, Joshi P, Zhao Y, Westphal H, McConnell SK (2017) Compensatory actions of Ldb adaptor proteins during corticospinal motor neuron differentiation. Cereb. Cortex 27:1686-1699.
Leone DP, Heavner WE, Ferenczi EA, Dobreva G, Huguenard JR, Grosschedl R, McConnell SK (2015) Satb2 regulates the differentiation of both callosal and subcerebral projection neurons in the developing cerebral cortex. Cereb. Cortex 25:3406-3419.
Srinivasan K, Leone DP, Bateson RK, Dobreva G, Kohwi Y, Kohwi-Shigematsu T, Grosschedl R, McConnell SK (2012) A network of genetic repression and derepression specifies projection fates in the developing neocortex. Proc. Natl. Acad. Sci. USA 109:19071-19078.
Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK (2008) The Fezf2-Ctip2 genetic pathway regulates the fate choice of layer 5 subcortical projection neurons. Proc. Natl. Acad. Sci. USA 105:11382-11387.
Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK (2008) The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 18:28-35.
Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas G, Grosschedl R, McConnell SK (2008) SATB2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57:364-377.
Chen B, Schaevitz LR, McConnell SK (2005) Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc. Natl. Acad. Sci. USA 102:17184-17189.
Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK (2008) The Fezf2-Ctip2 genetic pathway regulates the fate choice of layer 5 subcortical projection neurons. Proc. Natl. Acad. Sci. USA, in press.
Desai AR, McConnell SK (2000) Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development 127:2863-2872.
Frantz GD, McConnell SK (1996) Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron 17:55-61.
Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK (2008) The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 18:28-35.
McConnell SK, Kaznowski CE (1991) Cell cycle dependence of laminar determination in developing cerebral cortex. Science 254:282-285.




