RESEARCH:
Cell Fate, Biochemical Switches, and Xenopus Oocyte Maturation
What are oocytes?
 Oocytes are the postmitotic cells in the ovary slated to become fertilizable eggs. Fully grown (Daudin Stage VI) Xenopus oocytes are attractive brown-and-cream colored spheres about 1.2 mm in diameter. As cells go, they are gargantuan.
Oocytes are the postmitotic cells in the ovary slated to become fertilizable eggs. Fully grown (Daudin Stage VI) Xenopus oocytes are attractive brown-and-cream colored spheres about 1.2 mm in diameter. As cells go, they are gargantuan.
What is oogenesis?
The events that culminate in the production of an immature, G2-arrested oocyte are referred to as oogenesis.
Frog oocytes start out life as large but not gargantuan cells, and they go through a normal G1 phase and a normal S phase. They then pair up their homologous chromosomes and undergo meiotic recombination. Once that is completed they enter a growth phase where they grow ~250,000-fold in volume, mass, and protein and RNA content. Then they stop growing and enter what is essentially a G2-phase arrest, with a stockpile of tyrosine-phosphorylated, inactive cyclin-Cdk1 complexes.
This can be viewed as the default fate of the oocyte, and the oocyte can remain in this stable, arrested state for months.
What is oocyte maturation?
If the frog's endocrine system thinks the time is right for making tadpoles, it produces gonadotropins that induce the secretion of the steroid hormone progesterone by the epithelial follicle cells that surround each oocyte in the ovary. Progesterone acts on the oocyte through non-traditional, plasma membrane-associated receptors, causing the oocyte to be released from its G2-phase arrest and begin the process of oocyte maturation.
Biochemically, oocyte maturation begins with a drop in cAMP levels, activation of Aurora A, up-regulation of Mos translation and stability, activation of the Mos/MEK (MEK1)/MAP kinase (p42 MAPK or ERK2) cascade, and ultimately activation of cyclin-Cdk1. Active cyclin-Cdk1 drives the migration of the oocyte's huge nucleus (or germinal vesicle) to the animal pole of the cell, which causes a white spot to appear there. This is accompanied by germinal vesicle breakdown (GVBD), expulsion of the first polar body, and then the arrest of the oocyte in metaphase of meiosis II. At this point the oocyte is said to be mature.
The metaphase II-arrested state can be thought of as the induced fate of the oocyte, and it is a stable state too; the mature oocyte can stay arrested in meiosis II for a day or so, whereupon it will either be fertilized or die an apoptotic death. More info on this process can be found here and here.
Why should I care about this?
Although the details of oocyte maturation vary from species to species, there are a number of recurring themes. The technical advantages of Xenopus oocytes – their large size, easy accessibility, and their ability to reilably undergo maturation in vitro – make them a great model system, and Xenopus oocyte maturation has become a benchmark against which other maturation processes are compared. So even if you don't care about frogs, if you are interested in, say, human fertility, contraception, and reproduction, it's good to know about Xenopus oocyte maturation.
More generally, you can think of Xenopus oocyte maturation as an interesting example of a cell fate induction process, and cell fate induction occurs over and over again in development. The themes and principles underlying Xenopus oocyte maturation can be seen in other cell fate induction processes.
Since MAPK drives this all-or-none cell fate switch, is the activation of p42 MAPK all-or-none in character?
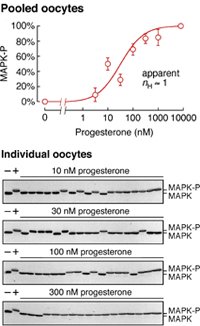 Not if you look at the average level of MAPK phosphorylation (a marker of activation) in populations of oocytes, as shown in the dose/response plot on the right. The response of MAPK is graded, well-approximated by a Hill function with an Hill exponent of 1.
Not if you look at the average level of MAPK phosphorylation (a marker of activation) in populations of oocytes, as shown in the dose/response plot on the right. The response of MAPK is graded, well-approximated by a Hill function with an Hill exponent of 1.
But if you look in individual oocytes, which is possible because an oocyte contains a lot of protein (~25 µg), you see something completely different. As shown in the blots on the left, each individual oocyte either has its MAPK 100% phosphorylated (in the upper band) or it has its MAPK 100% dephosphorylated. Thus the individual oocytes' responses are all-or-none in character.
What's the big deal? Aren't all protein kinases molecular switches?
In a sense they are. At the level of the individual moleule, a MAPK protein is either inactive or active (where by "active" we mean ~600,000-fold higher in activity than the inactive protein is, as shown by John Lew's lab here). But an oocyte contains around 180,000,000,000 MAPK moleules, so there is the potential for an almost infinitely-graded range of MAPK activity states.
So why does p42 MAPK always settle into one of two discrete activity states?
Bistability. The activity of p42 MAPK is controlled by a bistable circuit.
How does this bistable circuit work?
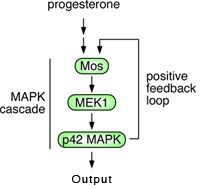 Mos activates MAPK via MEK1, and then active MAPK feeds back to activate Mos translation. This is a positive feedback loop, and positive feedback is essential for any bistable circuit.
Mos activates MAPK via MEK1, and then active MAPK feeds back to activate Mos translation. This is a positive feedback loop, and positive feedback is essential for any bistable circuit.
In addition, even in the absence of positive feedback the MAPK cascade generates an ultrasensitive steady-state response. By "ultrasensitive" we mean that the stimulus/response curve for MAPK activity as a function of Mos is well approximated by a Hill curve with a Hill exponent of 5 (as experimentally shown here). The combination of the ultrasensitive response of MAPK to Mos, and the positive feedback from MAPK to Mos, allows the system to be bistable and to generate an all-or-none response.
The bistability of the system, and the transition of the bistable system from off to on, can be understood through a rate-balance plot.
What is a rate-balance plot?
It is sort of like the supply/demand plots that economists use. They plot supply and demand as functions of price on one set of axes, and where the curves intersect, that is the equilibrium price of that commodity.
We plot the rates of Mos synthesis and Mos degradation as a function of the concentration of Mos present. Where the rate curves intersect, synthesis and degradation are balanced and the system is in steady state.
So what does the Mos/MAPK rate-balance plot look like?
First consider how the rate of Mos degradation should vary as the concentration of Mos varies. The simplest case, which we will assume here, is that Mos degradation increases linearly with the concentration of Mos present. This is shown as the blue curve below.
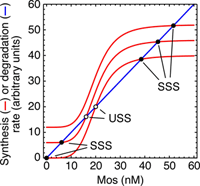 The synthesis rate curve is a bit more complicated. Here we have plotted three such curves, corresponding to three levels of the input stimulus progesterone. With the bottom curve (prog = 0) we imagine that the rate of Mos synthesis is zero if the Mos protein concentration is zero, and that the rate increases as Mos increases because of the positive feedback loop. Since MAPK activity is an ultrasensitive function of Mos, we assume that the rate of Mos synthesis is an ultrasensitive function of the Mos concentration as well. Hence the sigmoidal red curve. Here we have chosen it to be high enough to snake around the blue line.
The synthesis rate curve is a bit more complicated. Here we have plotted three such curves, corresponding to three levels of the input stimulus progesterone. With the bottom curve (prog = 0) we imagine that the rate of Mos synthesis is zero if the Mos protein concentration is zero, and that the rate increases as Mos increases because of the positive feedback loop. Since MAPK activity is an ultrasensitive function of Mos, we assume that the rate of Mos synthesis is an ultrasensitive function of the Mos concentration as well. Hence the sigmoidal red curve. Here we have chosen it to be high enough to snake around the blue line.
And why does this mean that the Mos/MAPK system is bistable?
The red curve intersects the blue curve at three points, which means there are three steady states for the system: an "off-state" with zero Mos (and zero active MAPK), an "on-state" with high Mos (and almost all of the MAPK active), and a steady state that is intermediate. The off- and on-states are stable (designated SSS in the plot above) and the the middle one is an unstable steady state (USS) or threshold. Above it the system will move up to the on-state and below it the system will move down to the off-state.
How does progesterone make the system switch from the off-state to the on-state?
As we add one unit of progesterone, we imagine that the basal (i.e. Mos-indepedent) rate of Mos synthesis increases. This shifts the red curve up vertically and changes the positions where the blue and red curves intersect. In particular, the off-state moves up and the threshold moves down. If we add another unit of progesterone, the red curve shifts up so far that the off-state and the threshold collide with and anhilate each other.
The input to the system (e.g. progesterone) can be thought of as driving the system through a saddle-node bifurcation, making a "saddle" (the USS) and a "node" (the off-state) disappear and forcing the system into the only remaining steady state, the on-state.
How does this bistable circuit affect the activity of Cdk1?
Active MAPK phosphorylates and activates the protein kinase Rsk2, which in turn phosphorylates and inactivates the protein kinase Myt1. Myt1 is an inactivator of Cdk1, so when Myt1 is turned off the Cdk1 can be turned on by an unopposed Cdc25C protein.
Cdk1 is embedded in its own set of positive feedback and double-negative feedback loops, which we will discuss further in our next installment. We have hypothesized, but not verified, that the two systems operate on different time scales: the p42 MAPK system, which uses tranlational positive feedback, a time scale of tens of minutes, and the Cdk1 system, which uses phosphorylation and dephosphorylation, a time scale of a couple of minutes. These dual-time systems can be very useful for switching quickly while still rejecting noise; click here for more discussion on this.
Once the positive feedback loop turns on, does it ever turn back off?
Not during oocyte maturation. You can see this in the rate balance analysis of the modeled system shown above. When you add progesterone, the red curves shift up and the system is forced into the only remaining steady state, the high-Mos state. But if you wash away the progesterone and shift the red curve back down, the stable high-Mos state does not disappear (it just shifts to the left slightly), and so the system would not be expected to flip back down to the low-Mos state. This rationalizes why the mature oocyte is locked in a stable steady-state with the positive feeback keeping the level of Mos and the activity of p42 MAPK high.
And, in fact, you can see the same thing in experiments on the actual biological system. If you wash away the progesterone from a mature oocyte, and it will stay mature, with high p42 MAPK and Cdk1 activities, for many hours. But if you perturb the positive feedback by blocking Mos synthesis, the irreversible activation of p42 MAPK and Cdk1 becomes reversible. Thus the positive feedback loops do generate an actively-maintained "memory" of the inductive stimulus progesterone (as shown by Wen Xiong; click here).
However, once a mature oocyte is ovulated and fertilzed, the positive feedback does turn back off. This happens because fertilization causes cyclins to be degraded, turning Cdk1 off, and causes Mos to be degraded, turning p42 MAPK off. Cyclin gets resynthesized during the ensuing mitotic cell cycles in the embryo, but Mos levels stay low.
Are bistable switches always irreversible, like a toggle switch?
 No. If the system is deterministic, the bistable switch can either be irreversible, like the p42 MAPK/Cdk1 system in oocytes, or hysteretic. As discussed in the next installment, the Cdk1 system in G2/M control (which does not operate together with a Mos/MEK/MAPK cascade) is hysteretic.
No. If the system is deterministic, the bistable switch can either be irreversible, like the p42 MAPK/Cdk1 system in oocytes, or hysteretic. As discussed in the next installment, the Cdk1 system in G2/M control (which does not operate together with a Mos/MEK/MAPK cascade) is hysteretic.
In either case, if the bistable system is noisy enough, it may be able to equilbrate between its two stable steady states, which is another kind of reversibility. The Collins lab's synthetic toggle switch is an example of this sort of behavior, as seen here. But the oocyte's toggle switch is strong enough to keep the cells from equilibrating between the "on" and "off" states.
Where can I read more?
Do bistable switches control other biological phenomena?
You bet. Bistability from transcriptional positive feedback is at the heart of the famous lambda bacteriophage lysis-lysogeny switch, and at heart of the famous beta-galactosidase induction system in E. coli. Muscle cell differentiation in culture depends upon another transcriptional positive feedback circuit. Fat cell differentiation in culture depends upon transcriptional positive feedback too.
Memories are probably maintained by cellular circuits in the brain that function as bistable switches. Epigenetic chromatin modification states are probably maintained by bistable circuits. Prion-mediated disease states are maintained by positive feedback loops that probably make the system bistable.
And in cell cycle regulation, a bistable switches control both the G1/S transtion and the G2/M transition. Much of the Ferrell lab's work over the last dozen years has focused on G2/M switch.
Is the Ferrell lab still interested in oocyte maturation?
Yep. After a bit of a hiatus, we recently we started up a project on the spatial organization of Cdk1 inactivation at the end of meiosis I. This project was inspired in turn by our work on bistability and trigger waves in Xenopus egg extracts which will be discussed in the next couple of installments.



