RESEARCH:
Bistable Switches and Cell Cycle Oscillations
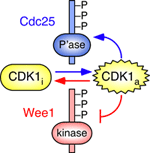 What is the "mitotic trigger"?
What is the "mitotic trigger"?
Early experimental studies showed that active Cdk1 could promote its own activation (by activating Cdc25) and inhibit its own inactivation (by inhibiting Wee1 and Myt1). Computational biologists (e.g. Tyson, Novak, and Thron) recognized that these feedback loops could constitute a bistable switch, aka the mitotic trigger.
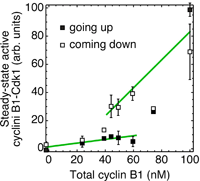 So is there a bistable trigger for mitosis?
So is there a bistable trigger for mitosis?
There is in Xenopus egg extracts, and presumably in many other cells and organisms as well. This was shown by experiments done in Jill Sible's lab (see here) and by Joe Pomerening in the Ferrell lab (right). When you dial up the cyclin concentration in an interphase extract, Cdk1 activity rises sharply once the cyclin exceeds about 70 nM. If you start with an M-phase extract and dial down the cyclin, the Cdk1 activity decreases and then falls more precipitously at a cyclin concentration of about 40 nM. Between 40 and 70 nM the system is bistable, with two alternative steady state.
What happens if you try to make a Xenopus extract do the cell cycle without a bistable trigger?
The oscillations fizzle out faster than normal; see here. And somatic cells have problems locking themselves into interphase after mitotic exit if you perturb the trigger by inhibiting Wee1; see here.
Note that intact Xenopus embryos require the trigger during their first post-fertilization cell cycle, but surprisingly they do not require it during cycles 2-12. So the cell cycle seems more robust in vivo than it is in extracts. See here.
In the previous installment, you mentioned that to generate bistability it is helpful for the feedback loops to include ultrasensitive responses. So do they?
Yes. The steady-state response of Wee1 to Cdk1 is ultrasensitive, with an effective Hill coefficient of 3.5. And the steady-state response of Cdc25C to Cdk1 is even more ultrasensitive, with an effective Hill coefficient of about 11. See this and this.
How are these huge Hill coefficients generated?
Wee1 and Cdc25C require multisite phosphorylation to be inactivated (Wee1) and activated (Cdc25C) by Cdk1. Multisite phosphorylation can generate ultrasensitivity, and multisite phosphorylation accounts for some of the ultrasensitivity observed. In the case of Wee1, competition with other higher affinity substrates appears to contribute to the ultrasensitivity as well.
Multisite phosphorylation is a recurring theme in cell cycle regulation, and in cellular regulation in general.
If you measured all the kinetic parameters needed for a basic model of the mitotic trigger, would you get a hysteretic response like the one seen by Pomerening and Sible? Or would you need to fudge some of the model's parameters?
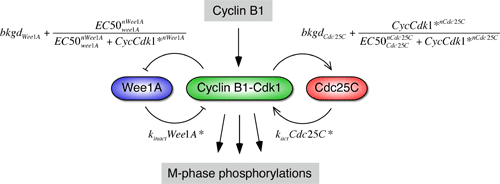 One simple yet reasonably realistic model of the mitotic trigger is shown schematically on the right. The most important simplification is that it assumes that Cdc25 and Wee1 are always at steady state with respect to the concentration of active Cdk1 in the system. This is probably not strictly true, but it allows one to derive a closed form expression that relates the steady-state level of Cdk1 activity to the concentration of cyclin B1 and all of the rate constants, EC50 values, and Hill exponents for the system.
One simple yet reasonably realistic model of the mitotic trigger is shown schematically on the right. The most important simplification is that it assumes that Cdc25 and Wee1 are always at steady state with respect to the concentration of active Cdk1 in the system. This is probably not strictly true, but it allows one to derive a closed form expression that relates the steady-state level of Cdk1 activity to the concentration of cyclin B1 and all of the rate constants, EC50 values, and Hill exponents for the system.
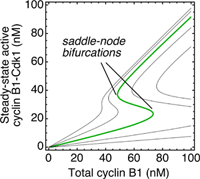 All of the parameters for the model have now been determined experimentally except for one, the ratio of Wee1 activity to Cdc25 activity in the system. The model produces a family of possible stimulus/response curves, shown on the right, with each curve corresponding to a different value for the activity ratio. For a reasonable value of the ratio (0.5) you get a bistable, hysteretic curve that agrees well with experiment.
All of the parameters for the model have now been determined experimentally except for one, the ratio of Wee1 activity to Cdc25 activity in the system. The model produces a family of possible stimulus/response curves, shown on the right, with each curve corresponding to a different value for the activity ratio. For a reasonable value of the ratio (0.5) you get a bistable, hysteretic curve that agrees well with experiment.
This agreement supports both the basic concepts underpinning the model – that the onset of mitosis corresponds to a traversal of a saddle-node bifurcation in a bistable control system built out of highly non-linear components – as well as the quantitative details of the model. See here for further discussion.
What's with the mirror-image postive and double-negative feedback loops?
Cdc25 and Wee1 are mirror-image proteins in many ways. The former is a phosphatase; the latter a kinase. The former activates Cdk1; the latter inactivates it. The former is activated by Cdk1, and the latter is inactivated by it. The former is inactivated by DNA damage checkpoint activation, and the latter is activated by it.
As mentioned in the discussion of oocyte maturation, interlinked positive feedback loops can be advantageous if the loops operate on different time scales (see this). But Cdc25 activation and Wee1 inactivation seem to happen at virtually the same time when Xenopus extracts go into mitosis. Is there some advantage to a mirror image relationship when the time scales of the loops are similar?
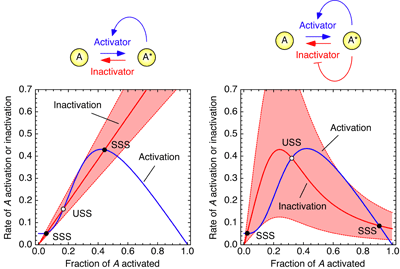 The answer is yes. It turns out that with a single positive (or double negative) feedback loop, bistability requires a fairly precise balance of protein concentrations. The mirror image arrangement allows the loops to work as a bistable switch over a much wider range of concentrations. This can be seen in rate balance plots, as shown on the right. If you have one loop, the system can tolerate a change of ~ ±30% in the concentration or activity of the inactivator protein. But with two loops, the system can tolerate a ~ ±300% change.
The answer is yes. It turns out that with a single positive (or double negative) feedback loop, bistability requires a fairly precise balance of protein concentrations. The mirror image arrangement allows the loops to work as a bistable switch over a much wider range of concentrations. This can be seen in rate balance plots, as shown on the right. If you have one loop, the system can tolerate a change of ~ ±30% in the concentration or activity of the inactivator protein. But with two loops, the system can tolerate a ~ ±300% change.
What about other cell cycle transitions? Are bistable switches involved there too?
Yes. The famous G1/S transition in budding yeast ("Start") happens when a transcriptional bistable switch is thrown. The same is thought to be true of the G1/S transition in mammalian cells. And in both cases, mirror image, interlinked positive and double negative feedback loops are involved. See here for a review.
The combination of positive feedback and bistability is a simple way of building all-or-none, hysteretic or irreversible responses out of intrinsically graded and reversible components. We suspect that nature commonly uses this type of regulation when decisive biological responses are needed.
How do you go from a bistable switch to an oscillator?
Add two things: cyclin synthesis, which drives the bistable trigger from interphase to M-phase, and a negative feedback loop where Cdk1 activation results in the activation of APC/C(Cdc20).
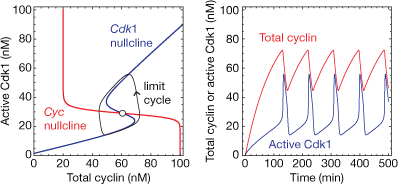 In dynamical terms, the result is a system that now possesses two steady-state stimulus/responses relationships or nullclines, one for Cdk1 as a function of cyclin and one for cyclin as a function of Cdk1. If the shape of this second (cyclin) nullcline is just right, the system will have a single steady state and the steady-state will be unstable. Instead of settling into a steady state, the system orbits it, essentially marching around the hysteretic stimulus/response relationship defined by the bistable trigger (the Cdk1 nullcline). This orbit around the hysteretic stimulus/response relationship is a stable limit cycle.
In dynamical terms, the result is a system that now possesses two steady-state stimulus/responses relationships or nullclines, one for Cdk1 as a function of cyclin and one for cyclin as a function of Cdk1. If the shape of this second (cyclin) nullcline is just right, the system will have a single steady state and the steady-state will be unstable. Instead of settling into a steady state, the system orbits it, essentially marching around the hysteretic stimulus/response relationship defined by the bistable trigger (the Cdk1 nullcline). This orbit around the hysteretic stimulus/response relationship is a stable limit cycle.
That cyclin nullcline looks extremely ultrasensitive. Is there some reason to assume that it APC/C(Cdc20) activity is such a switch-like function of the Cdk1 activity?
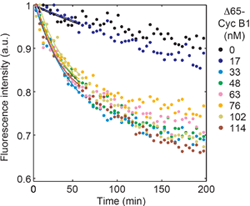 Yes. Qiong Yang showed that in Xenopus egg extracts the response is, in fact, ridiculously ultrasensitive, with an apparent Hill exponent of about 17. She measured this by monitoring the rate of securin-CFP degradation as a function of the concentration of non-degradable cyclin B1 added to the extract, as shown on the right. Tony Tsai showed that in extracts from later stage embryos, the apparent Hill exponent is even higher. See here and here.
Yes. Qiong Yang showed that in Xenopus egg extracts the response is, in fact, ridiculously ultrasensitive, with an apparent Hill exponent of about 17. She measured this by monitoring the rate of securin-CFP degradation as a function of the concentration of non-degradable cyclin B1 added to the extract, as shown on the right. Tony Tsai showed that in extracts from later stage embryos, the apparent Hill exponent is even higher. See here and here.
What accounts for the switch-like activation of APC/C(Cdc20)?
Probably a number of things contribute. Multisite phosphorylation of the APC/C complex, for one thing. Multisite dephosphorylation of the Cdc20 protein for another.
But while it is possible to achieve a response that is this switch-like through mechanisms like this, we suspect the response might actually be bistable, with positive or double negative feedback built into the APC/C(Cdc20) response. Béla Novák has hypothesized a plausible mechanism for this feedback.
Is it possible to sum this all up in a single, simple declarative sentence?
Sure. The cell cycle oscillator is built out of a bistable Cdk1/Cdc25/Wee1 toggle switch, with cyclin synthesis flipping the switch on and then APC/C(Cdc20) activation flipping the switch back off.
Do other biological oscillators work the same way?
Not necessarily. In principal you can build an oscillator from a long, time-delayed negative feedback loop without a bistable trigger. Michael Elowitz's Repressilator is an example of that type of oscillator, and the famous Goodwin oscillator model from the mid-1960's is another.
In Xenopus embryos, the synthesis of Cdc25A renders the bistable trigger of little consequence beyond the first couple of mitotic cell cycles. And in S. pombe, even though Wee1 is normally an essential gene, it becomes inessential in a system running off a minimal Cdk1-cyclin fusion protein. So even in cell cycle regulation, a bistable trigger is not always essential.
That said, it is striking how many biological oscillators do have a bistable trigger coupled to a delayed negative feedback loop. Think of pacemaker action potentials in the heart's AV node, or calcium oscillations in brain tissue. Nature appears to have settled on the same basic design over and over again. Further discussion of the advantatges of different oscillator designs can be found here.
Please sir, I want some more.
Here you go:



