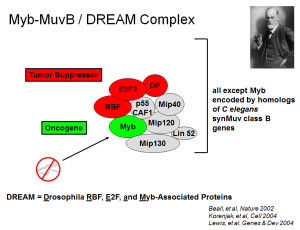
The Drosophila Myb protein was biochemically purified as part of a five-protein complex by Lolli Beall in the laboratory of Mike Botchan (UC Berkeley) in 2002. Two years later, additional work in the Botchan, Brehm, and Dyson laboratories identified a larger complex (Myb-MuvB/DREAM) in Drosophila embryos that also contained homologs of the retinblastoma (Rb) tumor suppressor family proteins and the E2F transcription factor family.
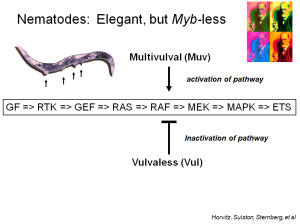
Quite remarkably, all but one of the proteins contained within this larger complex were encoded by the homologs of genes identified via synthetic multivuval (synMuv) mutations in the nematode worm C. elegans. The exception was Myb, which appears to have been deleted during the evolution of nematode worms. In brief, most nematode worms are hermaphrodites that have both male and female gonads. A single vulva (birth canal) is normally present on the external surface of the animal. Genetic screens conducted by Horvitz, Sulston, and their co-workers identified gain-of-function mutations in a highly conserved signal transduction pathway that resulted in worms with a multiple vulval (Muv) phenotype. Loss-of-function mutations in the same pathway resulted in a vulvaless (Vul) phenotype. Remarkably, the genes in the RAS pathway are frequently activated by mutations in a variety of human cancers.
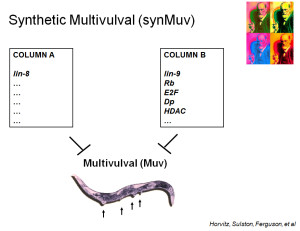
The same genetic screen yielded a worm in which loss-of-function mutations in two different genes were required for the Muv phenotype. This synthetic multivulval (synMuv) phenotype implied that the products of two different genes redundantly repress the RAS pathway. Additional loss-of-function mutations were then isolated that defined two classes of genes, synMuvA and synMuvB. Mutation of one gene from each class was required to produce the Muv phenotype.
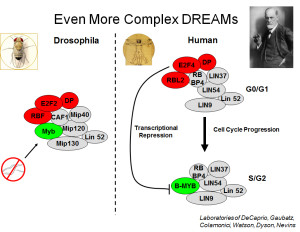
All of the proteins in the Myb-MuvB/DREAM complex of Drosophila (except Myb) were found to be encoded by homologs of the synMuvB genes. These results provided a biochemical explanation for the genetic observations. The proteins encoded by these genes are components of a machine that represses the transcription of other genes. Similar biochemical complexes were later identified in human cells by the Collamonici, DeCaprio, and Gaubatz laboratories. However, in human cell lines the MuvB core proteins were found to associate sequentially either with Rb-E2F-DP proteins or with Myb proteins as the cell division cycle progressed. Recent studies have shown that a similar switch between subcomplexes also occurs in somatic cells in Drosophila. It remains unknown whether the larger holocomplex is present in some normal human cell types.
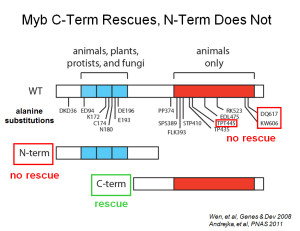
Recent work in our laboratory showed that a highly conserved animal-specific carboxy-terminal domain of Drosophila Myb is both necessary and sufficient for adult viability.
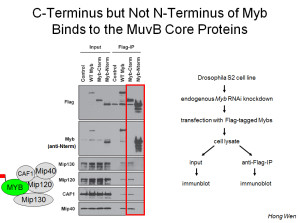
Furthermore, this conserved essential carboxy-terminal region is both necessary and sufficient for biochemical interaction with the MuvB core complex.
For the cognoscenti:
Drosophila E2F2 (in the complex) is the functional equivalent of human E2F4 and E2F5, whereas Drosophila E2F1 (not in the complex) is the functional equivalent of human E2F1, E2F2, and E2F3. The E2F proteins heterodimerize with the related DP proteins to form a functional DNA-binding transcription factor (sometimes referred to together as “E2F”). Drosophila RBF1 and RBF2 (either can be present in these complexes) are most closely related to human RBL1/p107 and RBL2/p130, rather than to RB itself (a more recent invention). Mip130 = LIN9. Mip120 = LIN54. Mip40 = LIN37. p55/CAF1 = RBBP4 = LIN53. RB proteins = LIN35 in C elegans. The “MuvB core” contains the homologs of LIN9, LIN54, LIN37, LIN53, and LIN52. In Drosophila, there is a paralogous testis-specific complex called tMAC.
For review articles, please see:
Lipsick. synMuv verite– Myb comes into focus. Genes & Development (2004)