

Mechanism:
Molecular Formula: C38H50N6O5 * CH4O3S
Molecular Weight: 766.96, 670.86
See below for Molecular Structure:
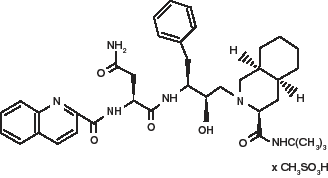
HIV protease cleaves viral polyprotein precursors to generate functional proteins in HIV-infected cells. The cleavage of viral polyprotein precursors is essential for maturation of infectious virus. Saquinavir mesylate, henceforth referred to as saquinavir, is a synthetic peptide-like substrate analogue that inhibits the activity of HIV protease and prevents the cleavage of viral polyproteins.
Indications and usuage:
Invirase in combination with other antiretroviral agents is indicated
for the treatment of HIV infection. This indication is based on results
from studies of surrogate marker responses and from clinical study that
showed a reduction in both mortality and AIDS-defining clinical events
for patients who recieved who recieved invirase in combination with other
antiretroviral drugs compared to patients who received drugs alone.
The recommended dosage is three 200 mg capsules daily. Invirase should be taken within two hours of completing a meal. Lower dosages have shown little or no therapeutic effect.
Precautions:
New cases of diabetes mellitus, worsening of pre-existing diabetes
mellitus and hyperglycemia have been reported during posmarketing surveillance
in HIV infected patients recieving protease-inhibitor treatment.
In some severe cases, diabetic ketacidosis has occured.
The safety profile of invirase in pediatric patients younger than 16 years has not been established.
Contraintradictions:
Fortovase is contrainicated for patients with known hypersensitivity
to saquinavir or its components. It should NOT be administered concomitantly
with terfenadine, casapride, astmiazole, triazolam, midazolam, or ergot
derivatives.
Potential Adverse Effects:
The majority of adverse events were of mild intensity. The most
frequently reported adverse events among patients receiving invirase were
diarrhea, abdominal discomfort, and nausea.