HPV-16 and HPV-18
Cervical Cancer
Killing 274,000 women annually, cervical cancer is the 2nd leading cause of death in women worldwide. In 2002, approximately 13,000 new cases of invasive cervical cancer and 4,100 deaths of women due to the disease were expected in the United States.
HPV infection
HPV infection is the primary causative agents of cervical cancer. A pool of evidence suggests that most cervical cancer squamous cell carcinomas and a large majority of adenocarcinomas share a common pathogenesis which involves infection with oncogenic high-risk HPV-16 and HPV-18. HPV-16 and HPV-18 account for two-thirds of all cervical cancers worldwide.
HPV high-risk verses low-risk
HPV-16 and HPV-18 have distinguishing biochemical and biological activities
compared to low-risk genital HPV types—HPV-6 and HPV-11. Differences
include:
- HPV-16 and HPV-18 oncoprotiens E6/E7 binding p53/pRB protein, respectively,
with a higher affinity than the corresponding HPV-6 and HPV-11 proteins
- enhanced potential of HPV-16 and HPV-18 E2 proteins to activate transcription
- ability of HPV-16 and HPV-18 to transform primary kertatinocytes in vitro
- E6/E7 proteins are sufficient and necessary for immortalization of human
keratinocytes
Oncogenic potential
E6 and E7 transforming oncoproteins of high-risk HPV genotypes play a crucial role in both the transformation and maintenance of the malignant phenotype. The interaction of E6 and E7 proteins with cell cycle-regulating proteins p53 and pRB leads to the disruption of normal growth regulatory mechanisms.
Because E6 and E7 oncoproteins are detected in a large proportion of HPV-positive cancer biopsies, it is suggested that the viral proteins could be ideal candidates for potential tumor-specific target antigens for cervical cancer immunotherapy.
HPV-16 verses HPV-18
HPV-18 is associated with;
- a more aggressive form of cervical cancer intraepithelial neoplasia
- invasive cervical cancer (adeno- and adenosquamous carcinomas)
- higher genome integration rate
- a greater likelihood of cancer recurrence and lymph node metastasis
- more efficient in the immortalization of epithelial cells
- more active promoter in primary human keratinocytes (HPV-18 P104 verses
HPV -16 P97 promoter)
Prevention:
abstinence, limiting number of partners, using a condom
yearly Pap smears
Treatment of cervical cancer:
- surgery
- radiation
- chemotherapy
http://www.cancer.gov/cancerinfo/pdq/treatment/cervical/patient/
More on the cancer.....
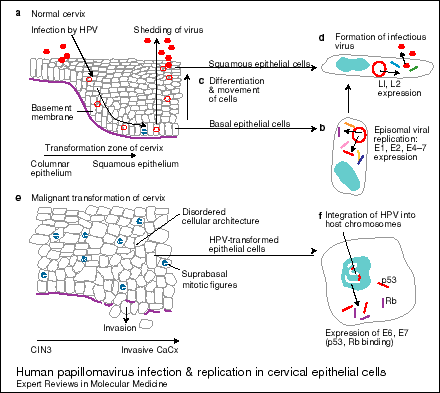
Figure 1. Human papillomavirus (HPV) infection and replication in cervical epithelial cells. (a) The normal cervix has a (narrow) transformation zone in which there is an abrupt transition from a columnar epithelium (sometimes via a metaplastic epithelium) to a squamous epithelium; HPVs are probably most infectious to cells that are close to this junction. (b) HPV viruses gain access to the basal epithelial cells of the cervix via the vagina (for example, during sexual intercourse), where they replicate episomally (outside the host chromosome in the nucleus) and express the (early) viral genes E1, E2, E4, E5, E6 and E7. (c) The infected basal cells, which show signs of cell disruption as a result of the viral infection, continue their differentiation and migration to the epithelial surface, where (d) the (now) squamous cells start to express the late HPV genes LI and L2. Infectious virus particles are formed and shed into the lumen of the vagina. (e) HPV infection (particularly with the high-risk types) can progress to: (1) HPV-induced mild dysplasia, (2) the final stages of cervical intraepithelial neoplasia (CIN3) and, eventually, (3) invasive cervical cancer (CaCx), when the basement membrane is breached by the cells, allowing local spread and also distant metastasis. (f) In transformed epithelial cells, HPV genes are integrated into the host chromosomes, with expression of (the oncogenic) E6 and E7 proteins, which bind to the tumour-suppressor proteins p53 and Rb (fig001smc).
http://www-ermm.cbcu.cam.ac.uk/smc/fig001smc.htm