|
March 2004 |
Herpes
Viruses
|
Humans and Viruses Human Biology Dr. Robert Siegel, Instructor |
|
Content Update 2004 Drug
Profiles
Pathogen
Cards
Melissa Sarah |
Introduction
The
Herpes virus family is composed of 8 members: 1.
Herpes Simplex-1 2.
Herpes Simplex-2 3.
Varicella-Zoster 4.
Epstein-Barr Virus 5.
Cytomegalovirus 7.
Herpes human virus-7 8.
Kaposi’s sarcoma associated herpes virus- 8.
For
a more complete summary of the entire family of herpes viruses, including
their physical properties and basic characteristics, check out the websites
made by students from Dr. Siegel’s previous Humans and Viruses classes: http://www.stanford.edu/group/virus/herpes/2000/herpes2000.html http://www.stanford.edu/group/virus/1999/inesicle/herpesvirus.html http://www.stanford.edu/group/virus/herpes/herpesvirusfamily.html We hope this webpage will serve as a concise update to the above webpages. This page includes: New findings, or summaries of recent herpes virus journal articles, treatment updates, featuring the drugs Valacyclovir and Docosanol, and interesting web sites for finding information about herpes viruses. Viral Profile:
HHV-6 and HHV-7 In
the last 20 years, Human Herpes Virus 6 and 7 have been discovered. As
compared to the other Herpes viruses, not much is known about these two. Both
viruses are very common the in US population (almost 100% infected) and most
individuals acquire these viruses by the age of two. The virus has been found
in the saliva and thought to be transmitted via saliva transfer. In children,
HHV-6 and HHV-7 cause rashes and sometimes fevers that go away on their own.
The rashes cannot be distinguished from the many other causes of childhood
rashes. Little more is known about HHV-7 and greater diseases it may cause. Pathogen."
Emerging Infectious Diseases vol 5 (3) May-June 1999 Update 2004
New Findings 2003-2004
Your jam as a
weapon against herpes
Suzutani
T, et al. Anti-herpesvirus
activity of an extract of Ribes nigrum L. Phytotherapy Research 2003; 17(6): 609 – 613 Researchers
in Japan found that blackcurrant, a common jam flavor in Europe and folk
medicine in Asia, has properties against HSV-1, HSV-2 and VZV. Blackcurrant prevented attachment of virus
to cells as well as viral protein synthesis.
Unfortunately, they didn’t purify out the exact element of
blackcurrant that was causing the decreased replication of herpes viruses.
Additionally, they found that blackcurrant had many toxic properties in vitro
but not in vivo. Perhaps what has the anti-herpes properties gets filtered
out of the human body as a toxin before ever reaching herpes-infected cells. Drugs prevent
the spread of genital herpes
Corey,
et al. Once-daily valacyclovir to reduce the risk of transmission of genital
herpes. When
acyclovir and valacyclovir first came out, it was thought that those with
reduced symptoms would also reduce the spread of HSV-2. This hypothesis was
shown to be valid and published in early 2004 in the New England Journal of
Medicine. What was interesting is that not only did valacyclovir reduce the
incidence of active disease and therefore spreading from those sores, but
viral shedding during periods of no sores was also reduced. Use of the
antiviral decreased transmission rates even when compared to occasional
condom use in the placebo group. That’s not to say that condoms don’t help,
but adding valacyclovir to the equation is very helpful. Overall,
valacyclovir taken daily reduced the amount of viral shedding and for how
many days virus was shed. Iron, herpes, and cancer
Simonart
T. Iron: a target for the management of
Kaposi's sarcoma? BMC Cancer. 2004 Jan 15;4(1):1. Iron
has been a way to understand why Kaposi’s Sarcoma doesn’t match perfectly
with the rate of HHV-8 virus infection in areas. It has been found that areas
that have greater volcanic clays in the environment have greater rates of
Kaposi’s Sarcoma. For instance, for individuals living close to Mount
Vesuvius in the Mediterranean and exposed to higher levels of iron have a
2-fold increased risk of developing KS than their neighbors. In Africa, where
KS usually affects the lower extremities, iron has been found in the feet
possibly acquired from the iron-rich soil. In HIV patients, iron metabolism
is affected and iron is unusually high in certain cell types. Iron has been
shown to increase cell proliferation as it is necessary for certain enzyme
activity. With iron possibly allowing for increased cell growth and assisting
HHV-8 in the development of cancer, iron-withdrawing drugs may be an
important tool to treat KS. New drug target for Varicella-Zoster Virus
Taylor, et al. Roscovitine, a cyclin-dependent
kinase inhibitor, prevents replication of varicella-zoster virus. J Virol.
2004 Mar;78(6):2853-62. Instead of targeting viral aspects of infection,
this group decided to find cell produced proteins that are required for
proliferation of HSV and VZV. They used Roscovitine, a molecule that blocks
the activity of several cyclin-dependent kinases. It has been shown that
various herpes viruses require cyclin-dependent kinases for replication. At
small doses, Roscovitine can block viral DNA replication without stopping
cellular replication. The inhibitor is also not toxic to most cells at this
concentration. Epstein-Barr and Multiple
Sclerosis Levin
LI, et al. Multiple Sclerosis and Epstein-Barr virus. JAMA. 2003 Mar
26;289(12):1533-6. In
2003, JAMA ran an article on the connection between Epstein-Barr and Multiple
Sclerosis, a disease currently without a concrete etiology. In other diseases
that have EBV has a possible cause like Burkitt lymphoma, nasopharyngeal
carcinoma and Hodgkin’s lymphoma, there’s an increase in antibodies to EBV
prior to onset of symptoms of disease.
EBV has been shown to possibly be involved in these cancers and the
increased antibody production before the symptoms has been pointed to as a
way to show a relationship between the viral infection and resulting disease. In
this study, old blood samples from people with MS were taken and examined for
levels of antibodies to EBV. It was found that years prior to developing MS,
these people had higher levels of antibodies as compared to individuals
infected with EBV but did not develop MS. Several
letters were written to state that many other factors could contribute to MS: Lily,
O. Epstein-Barr virus and risk of multiple sclerosis. JAMA. 2003 Jul 9;290(2):192 Tenser,
RB. Epstein-Barr virus and risk of multiple sclerosis. JAMA. 2003 Jul 9;290(2):192 Geldanamycin, a ligand of
heat shock protein 90, inhibits the replication of herpes simplex virus type
I in vitro. Li, Yu-Huan, Pei-Zhen Tao,
Yu-Zhen Liu, and Jian-Dong Jiang. Antimicrobial Agents and
Chemotherapy. March 2004, 48(3) p.
867-872. This
research presents a very recent discovery of an antibiotic that could prove
to be an effective antiviral.
Geldanamycin (GA), an antibiotic that can interfere with viral
replication, was “found to be active against HSV-I.” According to the authors, clinical trials
investigation of GA have started in the United States. Researchers involved with this study
attribute GA’s mechanism of “inactivation of cellular Hsp90.” Cellular Hsp90 allows HSV-I to disrupt the
cell cycle by inserting an extra checkpoint, inducing abnormal cell growth. Because GA “degrades” Hsp90, host cells
can proceed with cell growth as usual.
Such findings are innovative and exciting, as most antiviral products
and research revolve around degrading viral products, like DNA polymerase or
reverse transcriptase. It is a novel
approach to address cellular components in order to evade abnormal effects
from viruses. Herpes simplex virus. [in
children] Waggoner-Fountain, Linda,
M.D. and Leigh B. Grossman, M.D. Pediatrics in Review. 2004, 25 p. 86-93. Drs.
Wagonner-Fountain and Grossman give a comprehensive overview of herpes simplex
viral infection in infants, children, and adolescents. The authors present interesting
epidemiologic statistics about HSV; for example “between 20% and 40% of
infants infected with HSV are born preterm” and “in the United States,
approximately 75% of neonatal infections are due to HSV-2, with the remainder
due to HSV-1.” This data acknowledges
the importance of prenatal natal care and appropriate delivery of infants
with HSV infection. The authors
allude to the paradox that the most HSV viral shedding happens when a person
has a symptom, in this case a lesion.
However, transmission of HSV usually occurs when HSV is
asymptomatic. Thus it is often very
difficult to diagnose vertical transmission of HSV if the mother presents
with no HSV symptoms. Furthermore,
according to the authors, an infant without visible lesions “does not exclude
the diagnosis of neonatal HSV infection.” Cesarean
section is recommended by the authors if the mother has genital herpes, and
there are lesions in the birth canal.
It is also recommended not to use scalp monitors, which could increase
the chances of neonatal infection.
After the infant’s birth, healthcare workers must pay close attention
for signs and symptoms of neonatal infection. If a rash or lesion appears, a culture should be taken. “Signs of infection” to look for are: “lesion appearance, jaundice, seizures,
and respiratory distress.” If HSV is
found in the infant, acyclovir is administered intravenously. The authors reiterate that “with neonatal
HSV infection occurring as late as 4 weeks after delivery, parents and
physicians must be vigilant and carefully evaluate any rash or other symptoms
that may be caused by HSV…” Neonates
should be treated as immunocompromised when dealing with herpes viruses,
according to Waggoner-Foundtain and Grossman. Effect of topically
applied resveratrol on cutaneous herpes simplex virus infections in hairless
mice. Docherty, John J.,
Jennifer S. Smith, Ming Ming Fu, Terri Stoner, and Tristan Booth. Journal of Antiviral Research. January 2004, 61(1) p. 19-26. Researchers
conducting this study tested resveratrol as a topical cream to treat mice
infected with HSV. The authors found
that “25% resveratrol cream topically
applied two, three, or five times a day effectively suppressed lesion
development, whereas 12.5% resveratrol cream effectively suppressed lesion
formation when applied five times a day starting 1 hour after
infection.” Resveratrol, a natural
component of grape peels, like geldanamycin, is an example of antiviral
research that looks at mechanisms other than the classic “inhibition of DNA
synthesis by nucleoside analogues.”
The authors note that other important methods to fight viruses are: anti-sense mechanisms, inhibitors
targeting HSV helicase or primase, and inhibition of HSV ribonucleotide
reductase. The researchers conducted
the experiment comparing resveratrol to acyclovir topical cream and docosanol
topical cream (also known as Abreva).
Resveratrol and acyclovir performed well in the study, reducing lesion
size and preventing more outbreaks.
Docosanol was not as effective.
Another promising sign that resveratrol holds is that it showed no
side effects on mice; “none of the
animals showed any apparent signs of dermal toxicity such as erythema, scaling,
crusting, lichenification, or excoriation.”
Resveratrol
is an interesting compound in that it must affect more than one aspect of
HSV’s replication machinery. The
researchers in this study discussed how HSV could potentially affect the
ribonucleotide reductase that allows for HSV replication. However, resveratrol was also found to
regulate cell cycle by “inhibiting phosphorylation” which can cause abnormal
cell cycling. This important form of
treatment for herpes is undergoing further investigation. Tissue HHV6 and 7
determination in pediatric solid organ recipients – a pilot study. Gupta, M., F. Diaz-Mitoma,
J. Feber, L. Shaw, C. Forget, and G. Filler. Pediatric
Transplantation. December 2003, 7(6)
p. 458 This
article is a very interesting record of pediatric patients who are underwent
transplant, or did not undergo transplant but were immunocompromised. The researchers were analyzing the
relationship between HHV-6 and HHV-7 which are linked to organ rejection or
cytomegalovirus reactivation after the transplant. According to the authors, testing of “transplanted tissue for
herpes viruses may be more predictive of disease.” Thirteen patients were involved in the study; biopsies were obtained
to test for presence of HHV-6 or HHV-7, both before and after the transplant
(in patients receiving in organ). The
results noted that “only a poor correlation [exists] between HHV-6 serology
and the clinical picture,” indicative
of HHV-6’s ability to remain asymptomatic or latent. One very interesting patient had the
highest viral load, but was not a transplant patient, suggesting that it is
immunosuppression, not just receiving a foreign organ that may be HHV-6
positive, that corresponds to increased herpes virus susceptibility. Another patient had severe
gastrointestinal signs, and “PCR based diagnostics confirm[ed] the temporal
relationship between active HHV-6 and 7 infection and clinical events.” Immunization strategies to
block herpes simplex virus type 1 immunoglobulin G Fc receptor. Lin, Xiaoqing, John M.
Lubrinski, and Harvey M. Friedman. Journal of Virology. March 2004, 78(5) p. 2562-2571. This
study found that immunizing with gE fragments (proteins of HSV) could stop
HSV from avoiding immune system activity.
Normally, HSV can prevent antibodies from binding a specific receptor,
FcgammaR and thus avoid destruction by the host’s immune system. However, if the host is exposed to gE
protein fragments, “antibody bridging” can occur, and a receptor forms that
is like FcgammaR. These new receptors
can “use up” some of the HSV products that would normally block antibodies,
so “real” FcgammaR receptors remain open, which antibodies can bind to and
signal the immune system to destroy the infected cell. The “gE…fragment is a potential candidate
immunogen for future vaccine studies.” References
Corey,
et al. Once-daily valacyclovir to reduce the risk of transmission of genital
herpes. Docherty, John J., Jennifer S. Smith, Ming Ming
Fu, Terri Stoner, and Tristan Booth. Effect of topically applied resveratrol
on cutaneous herpes simplex virus infections in hairless mice. Journal of
Antiviral Research. January 2004,
61(1) p. 19-26. Gupta, M., F. Diaz-Mitoma, J. Feber, L. Shaw, C.
Forget, and G. Filler. Tissue HHV6 and 7 determination in pediatric solid
organ recipients – a pilot study. Pediatric Transplantation. December 2003, 7(6) p. 458 Levin
LI, et al. Multiple Sclerosis and Epstein-Barr virus. JAMA. 2003 Mar
26;289(12):1533-6. Li, Yu-Huan, Pei-Zhen Tao, Yu-Zhen Liu, and
Jian-Dong Jiang. Geldanamycin, a ligand of heat shock protein 90, inhibits
the replication of herpes simplex virus type I in vitro. Antimicrobial
Agents and Chemotherapy. March
2004, 48(3) p. 867-872. Lily,
O. Epstein-Barr virus and risk of multiple sclerosis. JAMA. 2003
Jul 9;290(2):192 Lin, Xiaoqing, John M. Lubrinski, and Harvey M.
Friedman. Immunization strategies to block herpes simplex virus type 1
immunoglobulin G Fc receptor. Journal of Virology. March 2004, 78(5) p. 2562-2571. Simonart
T. Iron: a target for the management of
Kaposi's sarcoma? BMC Cancer. 2004 Jan 15;4(1):1. Suzutani
T, et al. Anti-herpesvirus
activity of an extract of Ribes nigrum L. Phytotherapy Research 2003; 17(6): 609 – 613 Taylor, et al. Roscovitine,
a cyclin-dependent kinase inhibitor, prevents replication of varicella-zoster
virus. Journal of Virology. 2004 Mar;78(6):2853-62. Tenser,
RB. Epstein-Barr virus and risk of multiple sclerosis. JAMA. 2003
Jul 9;290(2):192 Waggoner-Fountain, Linda, M.D. and Leigh B.
Grossman, M.D. Herpes simplex virus. [in children] Pediatrics in Review. 2004, 25 p. 86-93. Interesting webpages Hopkins
HIV report (CMV eye infections) http://www.hopkins-aids.edu/publications/report/may03_4.html Current
projects dealing with CMV http://www.projectlinks.org/cytomegalovirus/ Immune
evasion and vaccine development CMV http://www.brown.edu/Courses/Bio_160/Projects1999/ies/hcmv.html Herpes
Academic Journal http://www.ihmf.org/journal/journal.asp Center
for Disease Control and Prevention’s site on EBV and Mono http://www.cdc.gov/ncidod/diseases/ebv.htm National
B virus Resource Center (resource on a dangerous zoonosis) http://www.gsu.edu/~wwwvir/index.html
Genital
herpes (CDC site) http://www.cdc.gov/std/Herpes/STDFact-Herpes.htm Varicella
disease (chickenpox) http://www.cdc.gov/hip/diseases/varicella/ Herpes
zoster (a secondary infection of varicella)
http://www.cdc.gov/nip/diseases/varicella/faqs-gen-shingles.htm Cytomegalovirus
(CDC site) http://www.cdc.gov/ncidod/diseases/cmv.htm International
Herpes Management Forum The
American Herpes Foundation http://www.herpes-foundation.org/ American
Social Health Association http://www.ashastd.org/hrc/index.html Drug Information
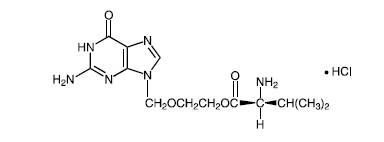
1. Valtrex
(Valcyclovir) General
Information
Valacyclovir
is the prodrug of acyclovir, a very common drug used to treat herpes
infection. Valacyclovir is used for
the treatment of HSV-1, HSV-2 (oral and genital herpes) and to a lesser
extent VZV (chickenpox/ shingles).
Valacyclovir is more bioavailable than plain old acyclovir meaning
that people can take less pills per day than acyclovir and still get all of
the benefits. Valacyclovir, with just one dose daily, has made it easier for
individuals with recurrent herpes infection to take suppressive doses of
acyclovir. Valacyclovir is licensed by GlaxoSmithKline under the name Valtrex®.
Mechanism
In
the gastrointestinal tract, Valacyclovir is converted to acyclovir. It then
needs the kinases produced by the herpes virus to become phosphorylated and
able to act in the cell. Alcyclovir, a guanine analog, works by stopping
viral DNA replication. Once acyclovir binds to the growing DNA strand, the
DNA polymerase cannot continue to add new nucleotides, hence stopping the DNA
replication. Valacyclovir is a bit more effective in HSV infections than in
VZV infections because HSV kinases are better at phosphorylating acyclovir
than those of VZV. Effectiveness
Who
can use it
Valtrex
can be used for the treatment of VZV, HSV-1 and HSV-2. It can also be used as
suppressive therapy for genital herpes, HSV-2. It should be noted that
valacyclovir is not a cure for any of these viral infections. Health people
and people with HIV and a CD4 count greater than 100 can use valacyclovir. How
much
Contraindications
Only
one: if a person is sensitive to valacyclovir or acyclovir. Precautions
People with kidney
problems or those with advanced HIV disease should not be given high doses of
valacyclovir. Valacyclovir can cause renal failure in individuals that
already have kidney problems. As for those with immune deficiency like in
HIV, valacyclovir can Thrombotic thrombocytopenic purpura/hemolytic uremic
syndrome with really high doses. Side
effects
According to the patient handout, valacyclovir can
cause “headache, nausea, stomach pain, vomiting, and dizziness.” Reference: Valtrex
prescribing information sheet. GlaxoSmithKline. http://us.gsk.com/products/assets/us_valtrex.pdf 2. "Abrexa
(docosanol)
General
information
Abreva
is a topical cream used to treat oral lesions, herpes labialis, related to
HSV infection. The cream’s active
ingredient is a 10% concentration of docosanol. It is available over the counter. Abreva®, or docosanol,
is a topical antiviral treatment for people with oral lesions related to
herpes simplex virus. It was developed by Avantir Pharmaceuticals, and is
manufactured by Smith-Kline Beecham. Mechanism
Docosanol
is an antiviral that prevents viral fusion to the cell’s membrane, so the
virus cannot gain entry into host cells. Effectiveness
According
to clinical trials and continued recording of Abreva’s efficacy, the cream
can reduce the length of time a person has an oral lesion by about one
day. The cream may also prevent
lesions from occurring when used prior to lesion appearance. Use of Abreva may also decrease the
severity and duration of symptoms, such as pain, tingling, or itching. Who
can use Abreva
Anyone
who experiences “cold sores or fever blisters” around the mouth may use
Abreva. Again, it is available
without a prescription. Please see
“Contraindications and Precautions” for more information. How
to use Abreva
The
cream should be “rubbed onto the lesion, gently and completely.” Contraindications
and Precautions
There
are no known contraindications or recommended precautions. However, Abreva has NOT been tested on
special groups, like the elderly, children, pregnant women or nursing
women. Pregnant or nursing women
should consult physicians before using Abreva, although animal testing showed
no birth defects when the mother used Abreva. There do not appear to be any problems associated with use by
the elderly or children. Abreva
should NOT be used on herpes virus- related lesions that are in regions other
than around the mouth. Abreva has NOT
been proven safe or effective for treatment of genital herpes lesions. Side
effects
According
to Avantis and Clinical Trial Watch, the possible side effect for people using
Abreva is headache. People receiving
a placebo during clinical trials for Abreva also noted headaches, so they may
not be related to docosanol use. Low
frequency side effects recorded are skin-related problems, such as: itchiness, dryness, acne, soreness,
swelling, redness. (Drug information online website). References
Center
Watch for Drug Clinical Trials: http://www.centerwatch.com/patient/drugs/dru627.html Drug
information online: http://www.drugs.com/index.cfm?pageID=0&htm=500219&type=cons&bn=Abreva&micr=medex#SXX1606 Avanir
Pharmaceutical Company website: http://www.avanir.com/product/product.php?ID=1 Abreva
photo, Avanir website: www.avanir.com/media/uploads/ products/abreva.gif |