Vivian's Webpage!!!
Timeline of Historical Events and Discoveries:
Through a mix of chance events and a deliberate search for the causes of
certain animal diseases, the parvoviruses were discovered. The diseases
caused by the parvoviruses such as erythema infectiosum have been known
since the beginning of the century. In 1959, Kilham and Olivier published
the account of a latent virus in rates that had been isolated using tissue
culture. This was the Rat Virus (RV) which later became known as a type
species of the genus Parvovirus. Once the size and morphology of RV had
been characterized, other parvoviruses in animals were identified by chance
and cell culture experiments.
It was not until 1975 during a screening of healthy blood donors for hepatitis
B surface antigens that human parvovirus B19 was discovered. B19 alludes
to the sample from which the parvovirus was first discovered. Initially,
B19 was identified as the causative agent of erythema infectiosum, a common
childhood rash found in outbreaks among schoolchildren during the winter
and spring months. Soon after, virologists discovered that parvovirus B19
was linked to fifth disease. Since then, B19 has been shown to be the causative
agent of many diseases. Please link to Clinical
Manifestations.
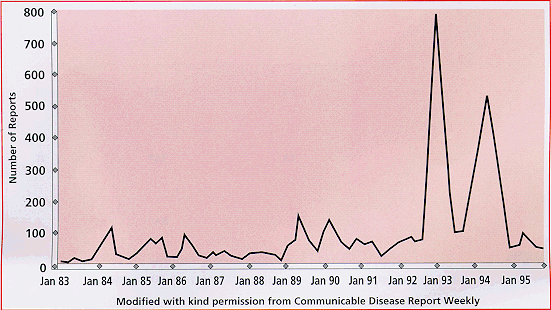
Finally, outbreaks of B19 occurred during January 1993 and 1994. Please
refer to figure 1 below (www.biotrin.ie/parvbook/b19book.htm#INTRO).
Figure 1: Report of Parvovirus B19

Viral Replication:
The replication of the parvovirus is closely linked to cellular replication. The virus replicates in the nucleus, initiates replication only after the host cell has completed the S phase of the cell cycle, and uses cellular DNA polymerases. Studies have shown that either the alpha or gamma DNA polymerase is utilized.
General Features:
1) Parvovirus DNA replication is accounted for
by a single-strand displacement model, similar to the one used to describe
adenovirus DNA replication. There are no lagging, discontinuous strands
involved in the DNA synthesis.
2) There are two stages of replication. Both
stages use terminal DNA sequences as primers. Thus, RNA or protein primers
or primases are not involved.
3) This model predicts site-specific cleavage
of intermediates during the replication.
Specific Steps of Replication:
The model for parvovirus replication is shown in Figure 2 below. The numbers
in parentheses correspond to the numbers in the figure.
Figure 2: Parvovirus DNA replication
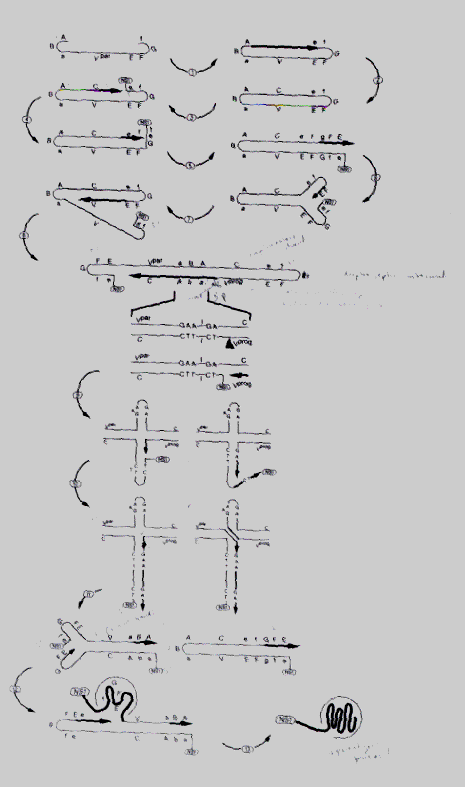
Key: ABa. 3'-terminal palindrome of virion strand; FGf. 5'-terminal palindrome of virion strand; e. 18-26-nucleotide sequence present in replicative intermediates but not in DNA of nuclease-treated virions; T, site-specific nuclease; V, virion strand; Vpar, parental virion strand; Vprog, progeny virion strand; C, complementary strand; X, site of possible topoisomerase action. (Fields, Bernard. Virology, 2180, 3rd edition, Philadelphia; Lippincott-Raven, 1996.)
The DNA replication of the parvovirus can be thought of as a modified rolling
hairpin. The ends of the viral genome are palindromic. In order to initiate
DNA synthesis, the 3' end of the viral genome folds back on itself to become
a primer (1). After some DNA synthesis along the complementary strand (2-5),
the 3' terminus of the complementary strand folds into a hairpin structure
to serve as a primer for further DNA replication (6). Replication continues
along the complementary strand (7). Next, an obligatory dimer duplex replicative
intermediate forms is formed. Within the palindrome regions lie small,
unpaired sequences which serve as cleavage sites for the NS1 nuclease.
The nuclease subsequently becomes covalently attached to the 5' side of
the nick (8). DNA replication continues along the viral progeny strand
(9-10). In the final steps (11-13), capsid protein recognizes the 3' hairpin
structure and packages the single strands. As can be seen in step 13, about
18-26 DNA nucleotides at the 5' end, along with NS1, are found outside
the capsid. These extra sequences will be cleaved off in the extracellular
environment or upon entry into the host cell.
The "Star" Researchers of the Parvovirus Family:
| Researcher Name | Contribution (s) |
| Cossart, Y. | Discovered human parvovirus. She reacted sera from blood bank donors with samples from hepatitis patients and noticed a series of false positive reactions, which when investigated further, turned out to be parvoviruses. |
| Filatov, N. | Formulated classification of red rashes of childhood. Parvovirus is on Filatov's list because it causes Fifth Disease of Childhood. |
| Kilham and Olivier | Discovered the Rat Virus (RV) which became the type species of the genus Parvovirus. |
| Ward, D. | Studies of minute-virus-of-mice. |
Table 1: Symptoms due to B19 Infection
Location or Time Period &nb sp; Clinical Symptoms Clinical Manifestation
| Nerves | neuralgic amyotrophy, plexus brachialis neuropathy |
| Pregnancy | fetal anemia, hydrops fetalis non-immune, abortion, stillbirth, neonatal death, and B19 infections in utero |
| Skin | erythema infectiosum (EI), fifth disease (for a description, link to www.stanford.edu/~megmath/parvovirus.html. |
|
Connective tissue and joints |
acute arthropathy |
| Blood and vessels | vaculitis, thromobocytopenia, hemophagocytic syndrome, transient aplastic crisis (TAC), petchial glove and sock syndrome, chronic infection in immunosuppressed individuals |
| Heart | congestive heart failure, myocarditis, AV-block and Adams Stokes attacks, pericarditis |
Further Notes on Clinical Manifestations:
Pregnancy: B19 infections in pregnant mothers are often overlooked because the disease is mainly asymptomatic. In some cases, infection may present with rash and joint complaints. For most pregnant women infected with B19, their child will be delivered normal. However, there is a significant chance of transplacental virus transmissions and fetal infection. After the mother experiences the onset of symptoms caused by B19 infection, she has the risk of losing her fetus up to 12 weeks after this initial onset. There is some evidence that B19 may produce teratogenic effects.
Skin: Please link to www.stanford.edu/~megmath/parvovirus.html
Blood and Vessels: About 68-100% of TAC cases are attributable to B19 infection. In 20% of these cases, infection is subclinical. Few patients report a rash.
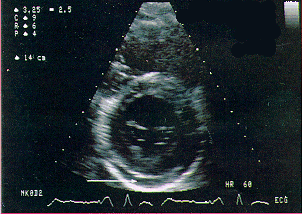
Heart: Heart complications due to B19 infection are rare. On a side note, B19 should be used as a differential diagnosis to borreolis in young and middle aged patients with acute AV-block. A picture of an AV-block is shown below in Figure 3. For further insight on differential diagnosis with B19, please link to www.biotrin.ie/parvbook/b19book.htm#INTRO.
Figure 3. Image of AV-block due to B19 infection
(www.biotrin.ie/parvbook/b19book.htm#INTRO):
Pathogenesis:
B19 virus can be detected in both blood and respiratory secretions five to seven days before a rash is developed and before antibodies can be detected in serum. The virus targets erythroid progenitor cells (lantern cells) in the bone marrow and spleen, which, when infected, undergo lysis. There is a resulting decline in the erythrocyte count as well as in lymphocyte, granulocyte, and platelet counts.
After the virus's incubation period of 10 days, fever, catarrhalia, and adenitis may start. Four days later, a rash may develop. The rash is believed to be an immune response because, simultaneously, B19-specific IgM antibodies can be detected in the host. B19-specific IgG antibodies can be detected a few days later. Also, the patient is no longer infectious via the normal transmission route a few days after the rash develops. Virus transmission via blood products is still possible. These persons without the blood group P antigen are naturally resistant to infection with B19 because it is its natural receptor. However, this occurrence is quite rare.
Diagnosis:
Although parvovirus infection can be quite apparent clinically, the virus's
inability to grow in standard cell culture systems has made widespread
laboratory testing of B19 quite difficult. However, detection of B19 IgG
and IgM allows for specific diagnosis of B19 infection. B19 infection can
be detected by the presence of IgM antibodies 14 days after the virus first
infects the individual. Right now, the most sensitive test to detect recent
infection is the IgM-antibody assay. Through either enzyme immunoassay
(EIA) or radio immunoassay (RIA), antibodies can be detected in about 90%
of cases by the third day after the onset of symptoms. In addition, tests
can also be done to test for the presence of viral DNA. Currently, the
most sensitive test for detecting the virus is nucleic acid hybridization.
These tests have suggested that B19 DNA was more likely to be present in
leukocytes than in serum. Moreover, detection of B19 IgG antibodies in
a patient's blood indicates protective immunity, which can last many years
in a majority of cases.
For more information on parvoviruses, you can
also check out the following web sites:
http://www.cdc.gov/ncidod/hip/abc/facts14.htm
http://www.cdc.gov/epo/mmwr/preview/mmwrhtml/00001348.htm
References:
Chorba et al "The Role of Parvovirus B19 in Aplastic
Crisis and Erythema Infectiosum (Fifth Disease)" Journal of Infectious
Disease, 154, 383-93, 1986.
Gillespie et al "Occupational Risk of Human Parvovirus
B19 Infection for School and Day-Care Personnel during an Outbreak of Erythema
Infectiosum" JAMA, 263, 2061-5, 1990.
Siegl, Bates, Berns, Carter, Kelly, Kurstak,
and Tattersall "Characteristics and Taxonomy of Parvoviridiae" Intervirology,
23: 61-73, 1985.
Thurn, Joseph "'Human Parvovirus B19: Historical
and Clinical Review" Reviews of Infectious Diseases. Vol. 10, No. 5, 1005-1011,
Sept-Oct 1998.
Webster and Granoff, Encyclopedia of Virology,
Volume 3. Academic Press.
Weiland et al "Parvovirus B19 Associated with
Fetal Abnormality." Lacnet, 1, 682-283, 1987.
For additional references, please refer to
www.stanford.edu/~megmath/parvovirus.html