Family Updates
In August of 1998, a live attenuated tetravalent Rotavirus vaccine was
licensed by the Food and Drug Administration. The vaccine was developed
at the National Institutes of Health (NIH) and is licensed to
Wyeth-Lederle Vaccines and Pediatrics. The vaccine is known as Rotashield
or RRV-TV. It contains a rhesus Rotavirus with serotype G3 specificity
and reassortant rhesus -human Rotaviruses with G1, G2, G4 specificity.
Therefore, a single vaccine provides immunity to the four most common
Rotavirus serotypes.
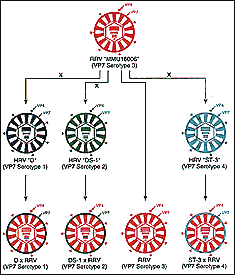 How the RRV-TV vaccine is made. Reprinted from Emerging
Infectious
Diseases, Volume 4 Number 4, Oct-Dec 1998.
How the RRV-TV vaccine is made. Reprinted from Emerging
Infectious
Diseases, Volume 4 Number 4, Oct-Dec 1998.
Four placebo controlled randomized clinical trails performed in
industrialized countries have demonstrated the efficacy of the vaccine.
It provides 50% protection against any diarrhea caused by Rotavirus and
more importantly, it provides about 70%-95% coverage against severe
diarrhea caused by Rotavirus.
The effects of the RRV-TV vaccine in unindustrialized countries are less
certain. Several factors influence its role: younger age of infection,
possible greater particle exposure, different strains of Rotavirus, poorer
nutritional status and interference by other diarrhea causing pathogens.
However, in a study in Venezuela, high rates of RRV-TV efficacy were
observed, approaching levels seen in studies done in industrialized
countries.
The vaccine is administered in three oral doses at 2,4, and 6 months of
age.
The first dose, however may be administered as early at 6 weeks of age
with a minimum interval between doses of 3 weeks. It is unknown whether
the vaccine will protect equally as well if less than three doses are
given. The vaccine is
currently priced at 10-30 dollars per-dose, a high price which may reduce
its feasibility in unindustrialized countries.
The vaccine is stored at room temperature, making it accessible even in
conditions where refrigeration is not possible. It is not known whether
the spread of vaccine virus, which has been observed, can lead to herd
immunity, protecting not only the child but also those who interact with
the
child. If herd immunity is a possibility, it may be possible to offer the
vaccination in limited numbers in unindustrialized coutries, while still
providing coverage to most.
Back

