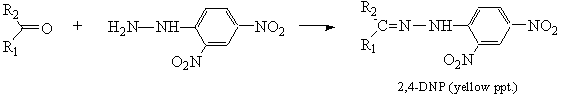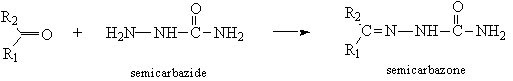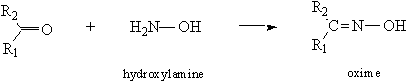Chemistry 130 course documents archive
Derivatives guidelines
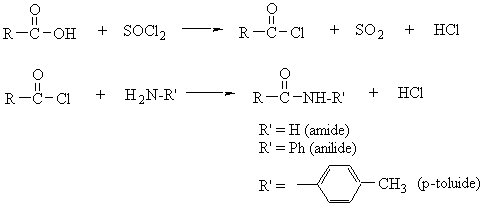
- Carboxylic Acids (Pasto 2nd ed. pp. 341–345)
- Amides
- Esters
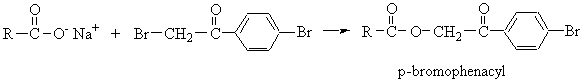
- Amides
- Alcohols and Phenols (Pasto 2nd ed. pp. 307–308)
- Urethanes
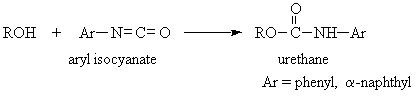
- Esters
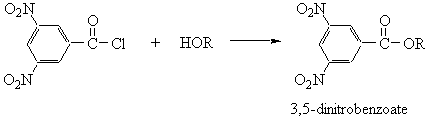
- Urethanes
- Aldehydes and Ketones (Pasto 2nd ed. pp. 328–333)
- Primary and Secondary Amines (Pasto 2nd ed. pp. 358–360)
- Sulfonamides
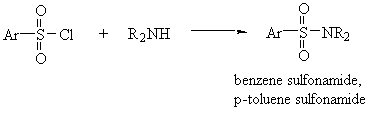
- Amides
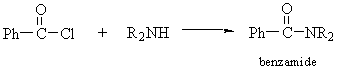
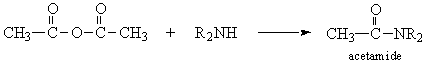
- Thioureas
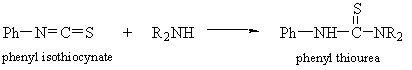
- HCl Salts
- Sulfonamides
- Tertiary Amines (Pasto 2nd ed. pp. 360–363) — form salts
- Methiodides
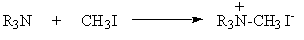
- HCl Salts
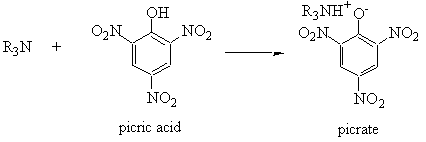
- Picrates*
- Methiodides
- Amides (Pasto 2nd ed. pp. 370–371)
- Hydrolysis
- Aryl Halides
- Nitration (Pasto 2nd ed. pp. 287, 300)
- Sulfonation → Sulfonamide (Pasto 2nd ed. pp. 300, 319)
- Side Chain Oxidation (Pasto 2nd ed. pp. 288, 300)
- Picrates*
- Aromatic Hydrocarbons
- Nitration (Pasto 2nd ed. pp. 286–287)
- Oxidation (Pasto 2nd ed. p. 288)
- Picrates*
- Aromatic Nitro Compunds
- Reduction (Pasto 2nd ed. p. 376)
- Nitration (Pasto 2nd ed. pp. 287, 377)
- Bromination (Pasto 2nd ed. pp. 319, 377)
- Alkyl Side Chain Oxidation (Pasto 2nd ed. pp. 288, 377)
- Esters (Pasto 2nd ed. pp. 349–351)
- Hydrolysis
- Aromatic Ethers
- Bromination (Pasto 2nd ed. p. 319)
- Sulfonation (Pasto 2nd ed. p. 319)
- Nitration (Pasto 2nd ed. p. 287)
- Picrates *
- Nitriles (Pasto 2nd ed. p. 373)
- Hydrolysis
* Pasto no longer recommends the preparation of picrate derivatives because picric acid and its complexes are explosive. If you have to make a picrate derivative, talk to your TA first about safety, and see Pasto and Johnson, Organic Structure Determination, on Reserve in Swain library for a general procedure. Picrates have been made successfully in the past.