During development, many young neurons migrate from their site of origin to the final position in which they differentiate. After their final cell division, the cells assume a bipolar morphology and migrate out through the intermediate zone and into the cortical plate, where they differentiate and form the six layers of the adult cortex. This outward or radial migration was demonstrated directly with thymidine birthdating studies in the early 1960’s. The electron microscopy studies of Pasko Rakic and in vitro studies of Mary Beth Hatten revealed that radial glia provide a substrate for radially migrating neurons. For many years, radial migration along glia was the only migratory pattern recognized in cortex and appeared sufficient to explain the neuronal movements known to occur during early cortical development. Evidence that cortical neurons do not follow strict radial pathways was provided initially by retroviral lineage studies from Chris Walsh and Connie Cepko, who suggested that clones of cells derived from single retrovirally-infected progenitors become dispersed tangentially over wide distances. We performed time-lapse imaging of living slices through the developing cortex revealed at least one mode of migration that contributes to tangential dispersion (O'Rourke et al., 1992). Bipolar cells, resembling young neurons, migrated tangentially through the cortical intermediate zone. Immunohistochemical studies confirmed the neuronal identity of these cells (O'Rourke et al., 1995, 1997), demonstrating that cortical neurons have a more diverse repertoire of migratory pathways than had been suspected previously. We now now that many of the tangentially migrating neurons are actually cortical interneurons, which are derived from the ganglionic eminences during development.
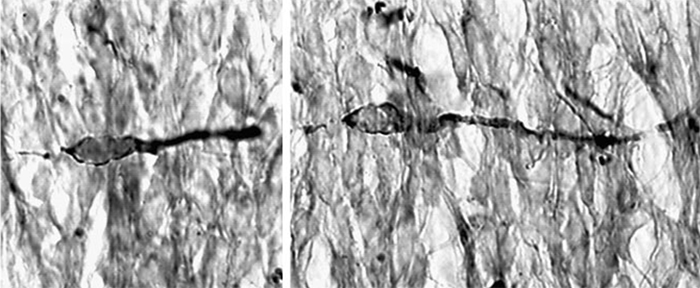
Doublecortin
Studies of the molecular mechanisms of neuronal migration have been greatly aided by the molecular cloning of human genes that are mutated in heritable forms of lissencephaly ("smooth brain") (Cahana et al., 2001; Aumais et al., 2001). Doublecortin (DCX) is a microtubule-associated protein that is mutated in X-linked lissencephaly (X-LIS), a neuronal migration disorder associated with epilepsy and mental retardation (Francis et al., 1999). Although DCX can bind ubiquitously to microtubules in nonneuronal cells, DCX is highly enriched in the leading processes of migrating neurons and the growth cone region of differentiating neurons. We have found that DCX/microtubule interactions are negatively controlled by Protein Kinase A (PKA) and the MARK/PAR-1 family of protein kinases (Schaar et al, 2004). In addition to a consensus MARK site, we identified a serine within a novel sequence that is crucial for the PKA- and MARK-dependent regulation of DCX's microtubule binding activity in vitro. This serine is mutated in two families affected by X-LIS. Immunostaining neurons with an antibody that recognizes phosphorylated substrates of MARK supports the conclusion that DCX localization and function are regulated at the leading edge of migrating cells by a balance of kinase and phosphatase activity.
The migration cycle
We then adopted an unbiased approach of correlative electron microscopy of neurons migrating through a three dimensional matrix, and to characterize the cytoskeletal events that occur as migrating neurons initiate saltatory forward movements of the cell nucleus (Schaar and McConnell, 2005). The formation of a cytoplasmic dilation in the proximal leading process precedes nuclear translocation. Cell nuclei translocate into these dilations in saltatory movements. Timelapse imaging and pharmacological perturbation suggest that nucleokinesis requires stepwise or hierarchical interactions between microtubules, myosin II and cell adhesion. We hypothesize these interactions couple leading process extension to nuclear translocation during neuronal migration.
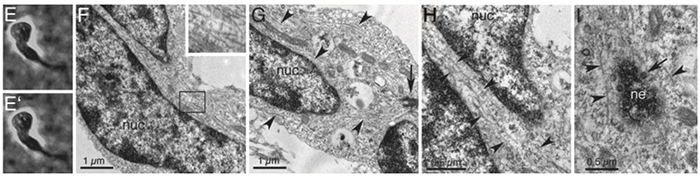
A model for saltatory neuronal migration: As the leading process extends, it makes adhesive contacts with the extracellular matrix. Following the growth of the leading process past this position, a cytoplasmic dilation forms distal to the cell nucleus. Prior to the onset of nuclear movement, the centrosome (red dot) moves into the forming dilation. Microtubules within the cell soma form longitudinal arrays and vacate the cell rear. The nucleus then translocates toward the centrosome along the longitudinal microtubule arrays. We postulate that an absence of microtubules at the cell rear triggers contractions mediated by myosin II, which generates a pushing force on the nucleus and serves to break adhesions at the cell rear. Nuclear movement stops as the nucleus enters the former location of the dilation, and the process begins again.
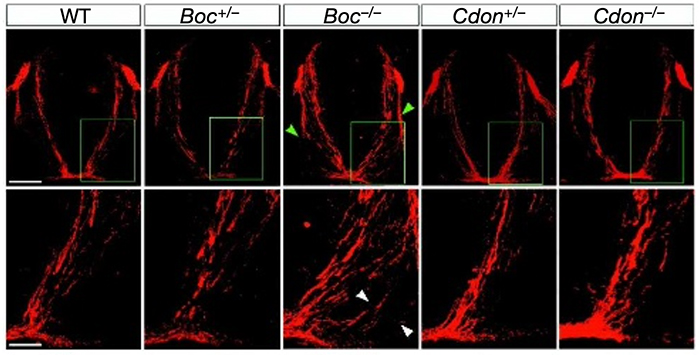
A role for Boc in axon guidance
Once a neuron reaches its final destination, it must elaborate dendrites and extend axons toward long-distance targets. Axon guidance is regulated by the expression of cues that can act as attractants or repellants either locally or over long distances. In the spinal cord, Sonic hedgehog (Shh) is secreted by the floor plate to control the generation of distinct classes of ventral neurons along the dorsoventral axis. Genetic and in vitro studies have demonstrated that Shh also later acts as a midline-derived chemoattractant for commissural axons. However, the receptor(s) responsible for Shh attraction remains unknown. We have found that two Robo-related proteins, Boc and Cdon, bind specifically to Shh and thus are candidate receptors for the action of Shh as an axon guidance ligand (Okada et al., 2006). Boc is expressed by commissural neurons and targeted disruption of Boc results in the misguidance of commissural axons toward the floor plate. RNAi-mediated knockdown of Boc impairs the ability of commissural axons to turn toward an ectopic source of Shh in vitro. Collectively our data suggest that Boc plays an essential role as a receptor for Shh in commissural axon guidance.
Adhesion and endocytosis in neuronal migration
During migration, young neurons must attach and subsequently detach from their substrate to facilitate migration, but little is known about the mechanisms cells use to release their attachments. We showed that the machinery for clathrin-mediated endocytosis is positioned to regulate the distribution of adhesion proteins in a subcellular region just proximal to the neuronal cell body. Inhibiting clathrin or dynamin function impedes the movement of migrating neurons both in vitro and in vivo. Inhibiting dynamin function in vitro shifts the distribution of adhesion proteins to the rear of the cell. These results suggest that endocytosis may play a critical role in regulating substrate detachment to enable cell body translocation in migrating neurons.
References:
Li J, Duarte T, Kocabas A, Works M, McConnell SK, Hynes MA (2014) Evidence for topographic guidance of dopaminergic axons by differential Netrin-1 expression in the striatum. Molec. Cell Neurosci. 61:85-96. PMID: 24867253.
Harwell CC, Parker PRL, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR (2012) Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron 22:1116-1126.
Shieh JC, Schaar BT, McConnell SK (2011) Endocytosis regulates cell soma translocation and the distribution of adhesion proteins in migrating neurons. PLoS ONE 6:e17802.
Cord BJ, Li J, Works M, McConnell SK, Palmer T, Hynes MA (2010) Characterization of axon guidance cue sensitivity of human embryonic stem cell-derived dopaminergic neurons. Molec. Cell Neurosci. 45:324-334.
Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre P, Tessier-Lavigne M, McConnell SK (2006) Boc is a receptor for Sonic Hedgehog in the guidance of commissural axons toward the ventral midline. Nature 444:369-373.
Gutin G, Fernandes M, Ornitz D, McConnell SK, Hébert JM (2006) FGF signaling generates ventral telencephalic cells independently of SHH. Development 133: 2937-2946.
Schaar BT, McConnell SK (2005) Cytoskeletal coordination during neuronal migration. PNAS 102:13652-13657.
Friedel RH, Plump A, Lu X, Spilker K, Jolicoeur C, Wong K, Venkathesh TR, Yaron A, Hynes M, Chen B, Okada A, McConnell SK, Rayburn H, Tessier-Lavigne M (2005) Gene targeting using a promoterless gene trap vector (“targeted trapping”) is an efficient method to mutate a large fraction of genes. PNAS 102:13188-13193.
Schaar BT, Kinoshita K, McConnell SK (2004) Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron 41:203-213.
Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK (2002) Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb. Cortex 12:702-709.
Aumais JP, Tunstead JR, McNeil R, Schaar BT, McConnell SK, Lin S-H, Clark GD, Yu-Lee LY (2001) NudC associates with Lis1 and the dynein motor at the leading pole of migrating neurons. J. Neurosci. 21: RC187.
Friocourt G, Chafey P, Billuart P, Koulakoff A, Vinet MC, Schaar B, McConnell SK, Francis F, Chelly J (2001) Doublecortin interacts with µ subunits of clathrin adaptor complexes in the developing nervous system. Molec. Cell. Neurosci. 18:307-19.
Cahana A, Escamez T, Nowakowski RS, Hayes NL, Giacobini MB, von Holst A, Shmueli O, Sapir T, McConnell SK, Wurst W, Martinez S, Reiner O (2001) Targeted mutagenesis of Lis1 disrupts cortical development and LIS1 homodimerization. Proc. Natl. Acad. Sci. USA 98:6429-6434.
Mulieri PJ, Okada A, Sassoon DA, McConnell SK, Krauss RS (2000) Developmental expression pattern of the cdo gene. Devel. Dynamics 219:40-49.
Mackarehtschian K, Lau CK, Caras I, McConnell SK (1999) Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cerebral Cortex 9:601-610.
Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet M-C, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J (1999) Doublecortin is a developmentally regulated, mictrotubule-associated protein expressed in migrating and differentiating neurons. Neuron 23:247-256.
Caric D, Gooday D, Hill RE, McConnell SK, Price DJ (1997) Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development 124:5087-5096.
O'Rourke NA, Chenn A, McConnell SK (1997) Postmitotic neurons migrate tangentially in the cortical ventricular zone. Development 124:997-1005.
O’Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnell SK (1995) Tangential migration of neurons in the developing cerebral cortex. Development 121: 2165-2176.
O'Rourke NA, Dailey ME, Smith SJ, McConnell SK (1992) Diverse migratory pathways in the developing cerebral cortex. Science 258:299-302.
McConnell SK (1985) Migration and differentiation of cerebral cortical neurons after transplantation into the brains of ferrets. Science 229:1268-1271.




