

1. Definition and Principle
Scintillators are materials that are able to convert high energy radiation such as X or gamma-rays to a near visible or visible light. They are widely used as detectors in medical diagnostics, high energy physics and geophysical exploration (ref. Knoll). Scintillators can be gaseous, liquid or solid, organic or inorganic (glass, single crystal, ceramics). Detectors based on scintillators (fig. 1) are essentially composed of a scintillator material, and a photodetector that can be either a photomultiplier tube (PMT) or a photodiode. The role of the photodetector is to convert the outcoming light of the scintillator to an electrical signal.
Photomultiplier tubes are the most common photodetectors, and are composed of a photocathode followed by a series of dynodes as shown in figure 1. The light photon strikes the photocathode, causing it to emit a photoelectron. The photoelectrons are focused onto the first dynode. This produces electrons that are multiplied at the second dynode, and again at the third, all the way down the chain.The amplified signal is then collected at the anode and passed out to the measurement circuits. The obtained electrical signal is proportional to the number of photoelectrons, Nphe.
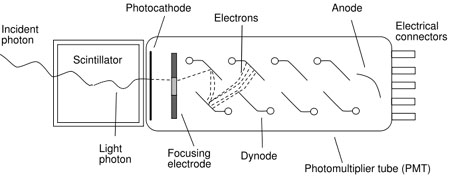
Figure 1: Schematic diagram of a scintillation detector comprising a scintillation material coupled to a photomultiplier tube.
2. Scintillator Mechanism
The physical phenomenon of scintillation is a complex process which can be divided into three main sub processes (fig. 2): Conversion, energy transfer and luminescence. The interaction of an electromagnetic radiation with the matter occurs through three mechanisms: Photoelectric effect, Compton scattering and electron-positron pair creation depending on the energy of the incident radiation. Photoelectric effect and Compton scattering are dominant mechanisms for low (below 100 keV) to medium energies (between 100 keV and 1 MeV) respectively. For energies above 1.02 MeV, the interaction of the radiation with the matter is governed by the electron-positron pair creation.
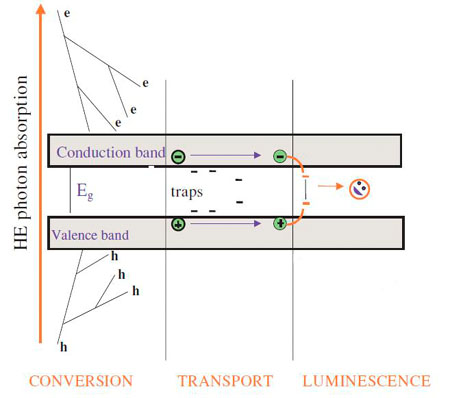
Figure 2: Scintillation mechanism. (Image by M. Nikl)
When the radiation is absorbed by the scintillator material, there is a creation of primary electron-hole pairs which generates secondary pairs by a cascade effect. When the energy of the electronic excitations becomes below the ionization threshold, the thermalization takes place. At the end of this stage, all the electrons are at the bottom of the conduction band and the holes at the top of the valence band. This first step is concluded within less than a picosecond.
After the thermalization stage, the free electron hole pairs migrate through the material so that they transfer their energy to the luminescent centers. The energy transfer is very quick and can be done in 10-12 to 10-8s. Once the energy transfer is done, the last stage of scintillation, namely the luminescence, takes place. The duration of luminescence depends on the luminescent centers and can take more than 10-10s.
The energy of the emitted photon is an important parameter which allows the differentiation between radioisotopes. Indeed, the energy of the emitted photons is related to the energy of the incoming radiation. Depending on the nature of this relationship, one may be able to determine the radioactive source. In the case of scintillator materials, the photoelectric effect is to be favored because the complete incoming radiation is absorbed by the medium. The Compton effect generates photons with less energy which leads to error sources. In order to enhance the probability of the photoelectric effect to occur, materials with high atomic number Z and high photoelectric fraction are preferred. The photoelectric fraction is the proportion of incoming photons that interact with the matter by photoelectric effect.
3. Characteristics of scintillators.
- Light yield (photons/MeV): Number of emitted photons per absorbed energy.
- Energy resolution (%): Ability of a material to discriminate between two radiations of slightly different energies.
- Decay time (s): Kinetics of the light response I(t) characterized by tau.
- Afterglow: Residual light output occurring after the primary decay time of the main luminescent centers.
- Stopping power: Attenuation coefficient of the absorbed radiation, for a given thickness of a material.
4. Fabrication of scintillators.
Until recently, inorganic scintillators are in the form of single crystals. These are typically produced by growing techniques from the melt such as Czochralski or Bridgman-Stockbarger method.
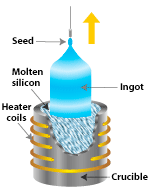
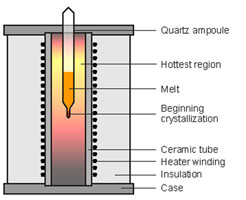
Figures 3 & 4. Czochralski apparatus (left) and Bridgman-Stockbarger furnace (right).
The Czochralski apparatus is shown in figure 3. One attaches a seed crystal to the bottom of a vertical arm such that the seed is barely in contact with the material at the surface of the melt. The arm is raised slowly, and a crystal grows underneath at the interface between the crystal and the melt. Usually the crystal is rotated slowly, so that inhomogeneities in the liquid are not replicated in the crystal. Based on measurements of the weight of the crystal during the pulling process, computer-controlled apparatuses can vary the pulling rate to produce any desired diameter. As the seed is extracted, the material solidifies and eventually a large circular boule is produced. Czochralski method is usually used for materials with high melting point.
The Bridgman-Stockbarger apparatus is shown in figure 4. The method involves heating a polycrystalline material in a sealed ampoule, which has a cylindrical shape with a conical lower end. Heaters maintain the molten state. As the ampoule is slowly lowered into a cooler region (blue region), a crystal starts growing in the conical tip. The ampoule is lowered at a rate that matches the growth of the crystal, so that the interface between crystal and melt is always at the same temperature. The rate of moving the ampoule depends on the temperature and the material. When done successfully, the entire molten material in the ampoule grows into a single large crystal. A layer of impurities grows at the interface between melt and solid as this surface moves up the melt, and the impurities become concentrated in the higher part of the crystal. This method is well suited for materials with low melting point and sensitive to air such as Strontium Iodide.
>>Return to Background & Fundamentals page<<