Numerical simulations of practical combustion systems, such as furnaces, pool fires and even engines, often lack an adequate description of the involved chemistry. The reason is, most of all, that real fuels are composed of many single components, involving, for instance, aliphatic, aromatic, and alkylated aromatic components, but also cyclo-parrafins and possibly oxygenated components. For some of those components, detailed chemical kinetic mechanisms are already available. However, these mechanisms tyically consist of thousands of reactions and as many as one thousand chemical species.
It is clear that it is impossible to model the true chemistry of a realistic fuel. Hence, for numerical simulations, appropriate surrogate fuels have to be used, which consist of one or more chemical components and reproduce the behavior of the real fuel as well as possible. The present work aims at the development of a library of chemical mechanisms for surrogate fuels suitable for practical numerical simulations. This has two major axis: the systematic reduction of the detailed mechanisms to the smallest possible size that still describe the fuel chemistry accurately, and the compilation of multi-component chemical mechanisms from available single-component mechanisms.
Ignition delay times for stoichiometric mixtures of iso-octane and n-heptane of varying octane numbers at 40 bars. Symbols are experiments by Fieweger et al, solid black lines are results using a detailed mechanism for Primary Reference Fuel from LLNL (Curran et al, 1000 species), colored dashed lines are results using a reduced mechanism (340 species)
Click image
to for enlarged version.
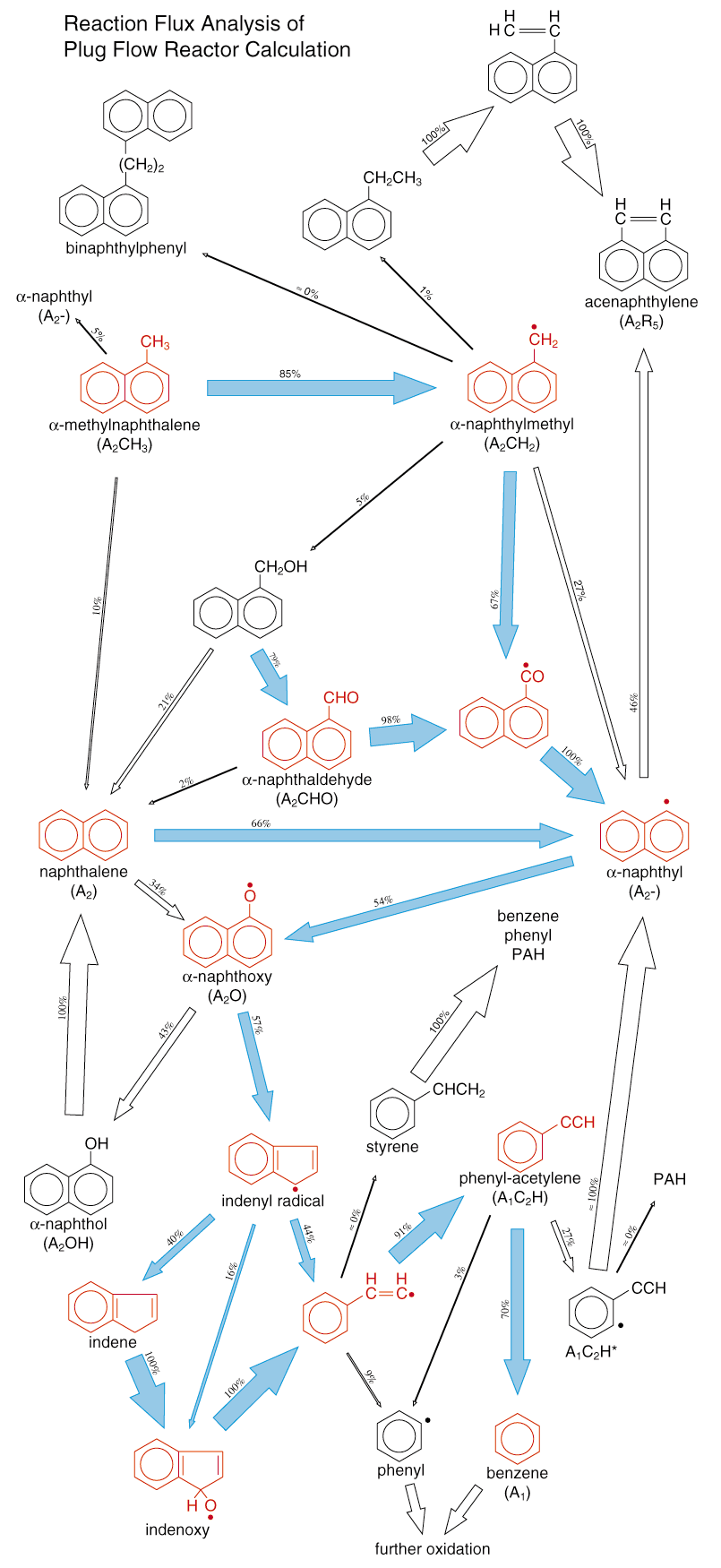
An example of surrogate fuel for Diesel engine simulations is a mixture of long-chain aliphatic species such as n-heptane or n-decane, and aromatic compounds like alpha-methyl-naphthalene. Aromatics are well-known knock inhibitors and are used as additives to supply high-octane unleaded gasoline. However, they contribute significantly to pollutant formation like soot and thus, have to be modeled with care. The picture represents the major species and reaction pathways appearing during the oxidation of alpha-methyl-naphthalene and shows the complexity of the chemical processes taking place during hydrocarbon oxidation.
Click image
to for enlarged version.