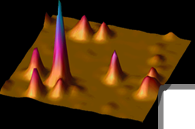
Poly(acrylamide) Gels for Single-Molecule Imaging and Biophysics
3-D Localization of Single Small Fluorophores Undergoing Restricted
Brownian Motion
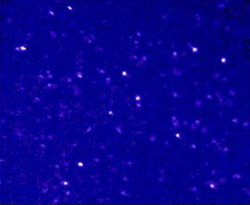
Figure 1: Fluorescence image (25 micron
x 20 micron, 1 s exposure) of single nile red molecules
in a PAA gel.
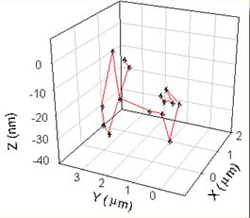
Figure 2: 3-D motion determined
by our TIR technique. |
R. M. Dickson, S. Kummer, and W. E. Moerner
Since our initial observations of single molecules in 1989,
the studies performed by many different groups have significantly
furthered the understanding of single molecule behavior in low
temperature glasses and crystals. In order to obtain analogous
in formation from the behavior of single molecules at room temperature,
we are employing near-field and far-field microscopic techniques
to better understand biological systems. Although most room temperature
single molecule studies have been performed in polymer hosts
or on surfaces, we have developed exciting new techniques that
have allowed us to study single molecules in aqueous solutions
(Please see our recent Science article). Our studies have
opened up an entirely new class of systems for physical and in
vitro biophysical single-molecule studies. Currently we are
investigating photophysical and biological properties of individual,
singly-labeled proteins and their environmental interactions.
By employing the exquisite local environmental sensitivity that
single molecule studies enable, we are deciphering biomolecular
mechanisms that are obscured in bulk biological studies.
By employing water-based polyacrylamide (PAA) gels, we have
been able to restrict Brownian motion of individual dye molecules
and singly-labeled proteins. The random gel matrix enhouses many
water-filled cavities ("pores") through which the molecules
can move. With these techniques we have directly observed the
translational motion of small dye molecules in high acrylamide
concentration gels and the motion of small proteins in lower
concentration gels. The TIR geometry provides a strongly varying
optical intensity in the axial direction, which allows determination
of not only the xy position, but also the Z-position of the molecule
by its overall brightness (see the lower panel in the figure
below). As the acrylamide concentration is increased, the molecular
motion becomes increasingly restricted; this means that large
single proteins can be trapped in individual gel pores and studied
for long times in aqueous environments. This represents a significant
advancement over previous methods which were only able to detect
the presence of single proteins in solution as the protein diffused
(quite rapidly due to Brownian motion) through the focal spot
of the microscope. Our techniques allow for long time study of
proteins and their behavior in solution (see figure 3 below).
Studies of the naturally fluorescent Green Fluorescent Protein
(GFP), for example, have identified both blinking and optical
switching behavior on the single molecule level (please refer
to our recent article in Nature). We have many other experiments
in progress and hope to extend both the gel based studies and
other solution based biophysical techniques to the understanding
of a wide range of biological systems.
Figure 3: 20 micron x 20 micron image of
individual Cy-3 labeled BSA (Bovine Serum Albumin)
proteins in a polyacrylamide gel.

Figure 4: Dr. Rob Dickson at the TIR microscope.
|
Recent Publications
- "Three-Dimensional Imaging of Single Molecules Solvated
in Pores of Poly(acrylamide) Gels," by
R. M. Dickson, D. J. Norris, Y.-L. Tzeng, and W. E. Moerner,
Science, 274, 966-968 (1996).
- "On/Off Blinking and Switching Behavior of Single Green
Fluorescent Proteins," by R.M. Dickson,
A.B. Cubitt, R.Y. Tsien, and W.E. Moerner, Nature, 388, 355
(1997).
|