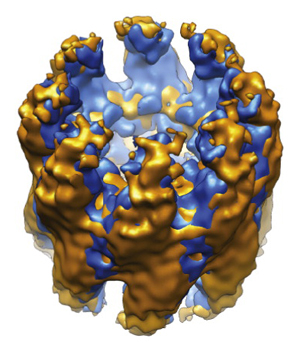
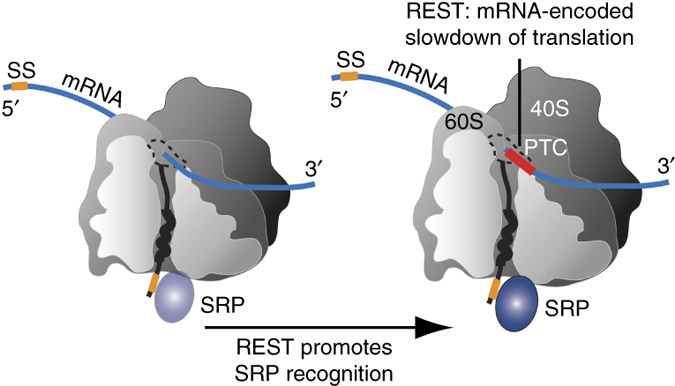
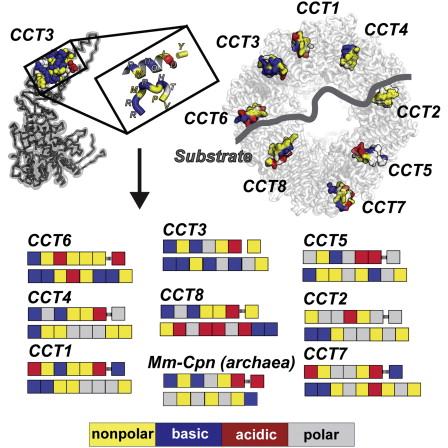
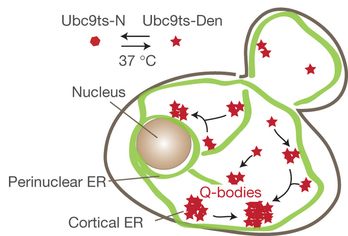
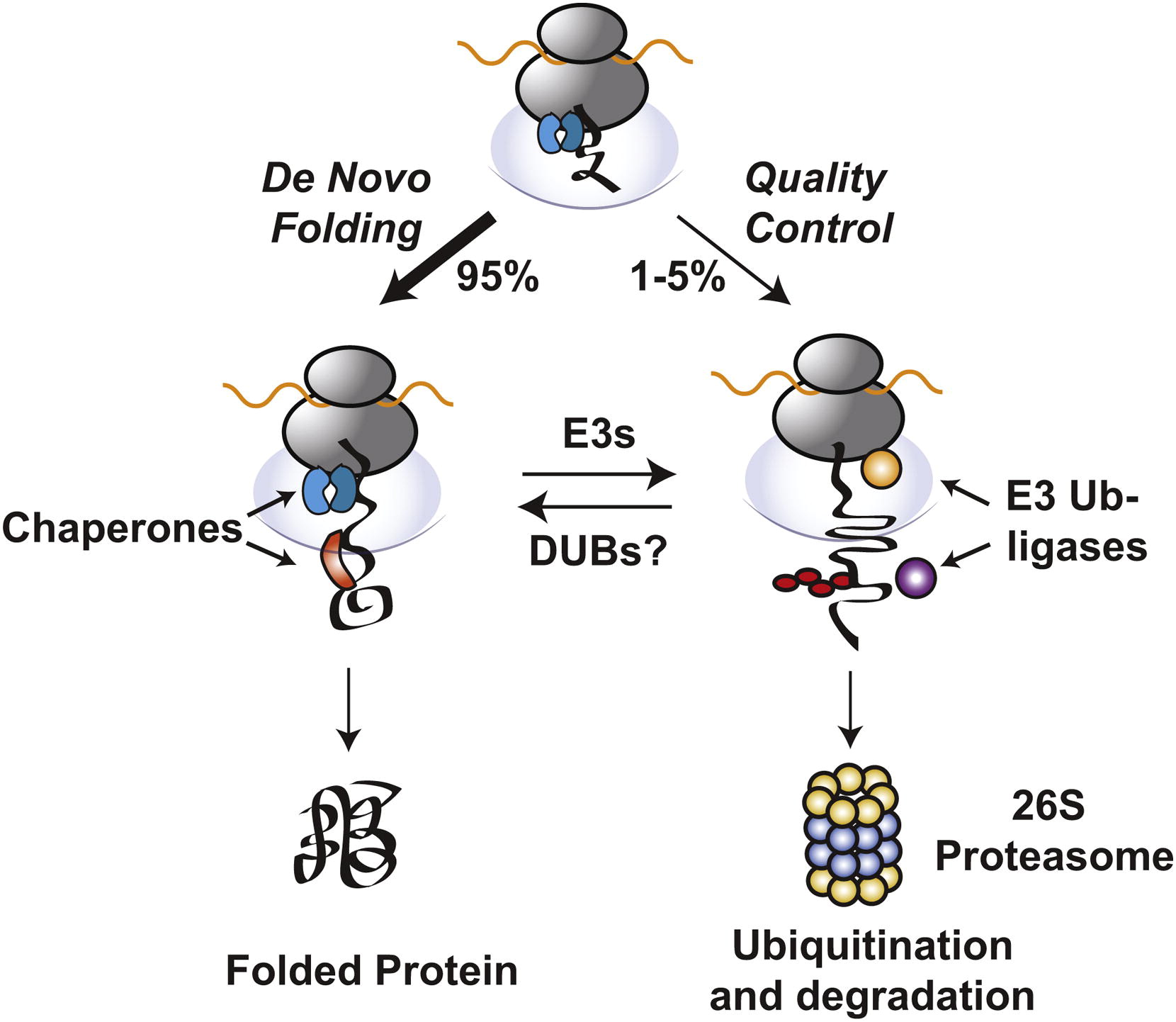
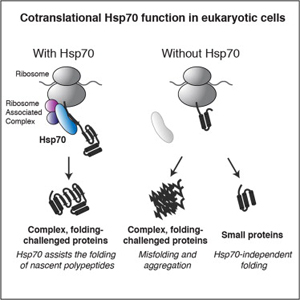
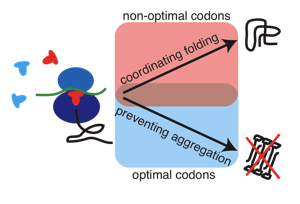
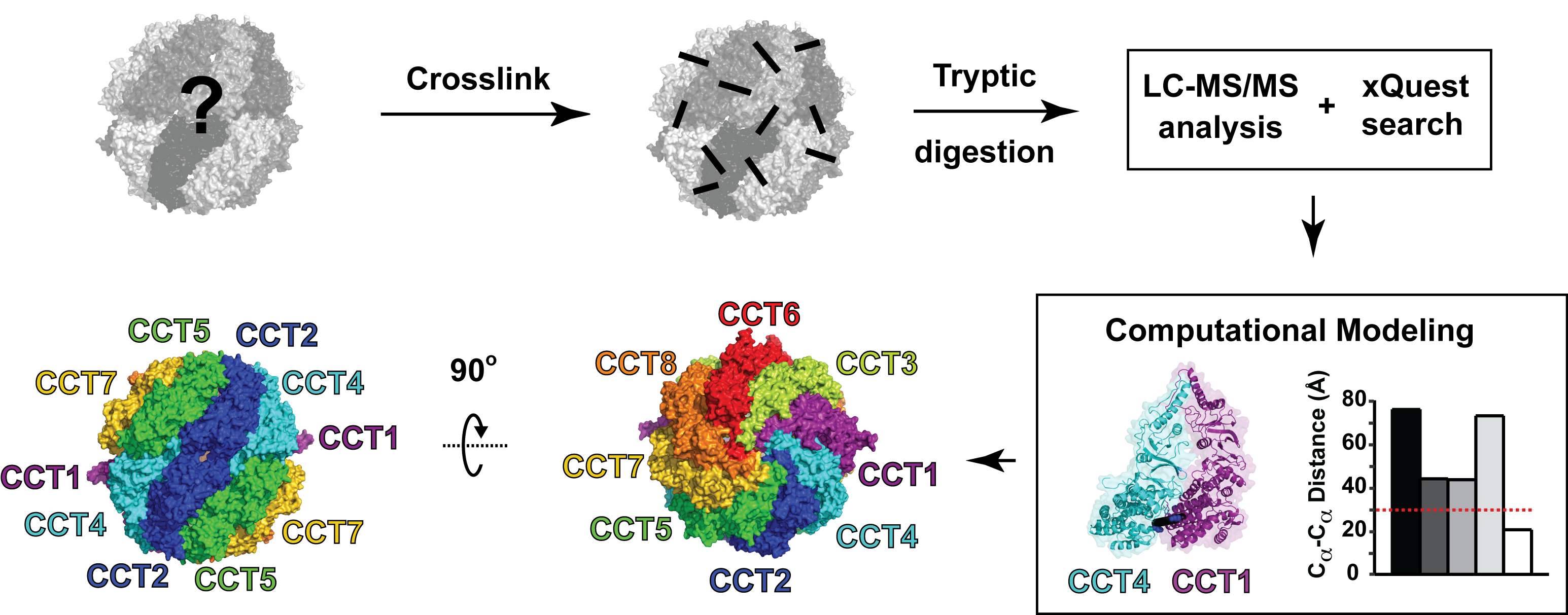
Molecular chaperones and protein folding in the cell
The long term goal of our research is to understand how proteins fold in living cells. The Frydman lab uses a multidisciplinary approach to address fundamental questions about molecular chaperones, protein folding and degradation. In addition to basic mechanistic principles, we aim to define how impairment of cellular folding and quality control are linked to disease, including cancer and neurodegenerative diseases and examine whether reengineering chaperone networks can provide therapeutic strategies.