The Ferrell lab is working to understand the design principles of biochemical switches, timers, and oscillators, especially those that control the cell cycle. We make use of quantitative experimental approaches, modeling, and theory.
* * * NEWS * * *

Mar 2024: Third paper of the month (!): Shixuan Liu's work on hormonal signaling in the gray mouse lemur was just published in Nature Communications. Shixuan made use of the mouse lemur cell atlas (or Tabula Microcebus), which was produced by a consortium of 150+ researchers organized by our colleague (and Shixuan's co-mentor) Mark Krasnow, and which is available on bioRxiv (Ezran C, Liu S et al.), to obtain a global view ofstor hormonal signaling in this organism. This is a wide-ranging paper. Some of the highlights include the finding that the mouse lemur's signaling system is substantially more similar to the human system than the rodent hormonal signaling system is; that virtually all cells "hear" more hormonal languages than they "speak"; and that despite the hierarchical structure of the famous hypothalamus/pituitary/adrenal axis, overall hormonal signaling is densly connected, highly distributed, and decentralized. But we're just scratching the surface--have a look at the paper to get the full story. And congrats to Shixuan and all the brilliant and generous scientists who made this possible.
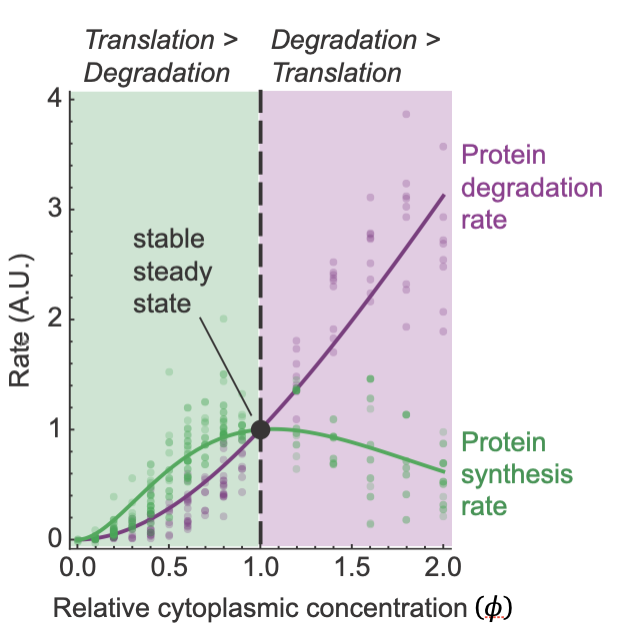
Mar 2024: Another fascinating paper awaits! From Yuping Chen, Jo-Hsi Huang, and Connie Phong, this paper focuses on the question of why protein concentration is as high as it is in cells, but no higher? In 2011 Ken Dill conjectured that at a 1x protein concentration, the speed of key biochemical reactions might be maximized. Below 1x, dilution of an enzyme and its substrate makes the process run slower, and above 1x the increase in cytoplasmic viscosity makes it run slower. This is an attractive idea, but how would you test it experimentally?
Well, Yuping and his colleagues tested it by making use of Xenopus egg extracts, which can be diluted easily, and which can also be concentrated as high as 2x. And the processes they chose to examine were protein synthesis and protein degradation, which not only might be affected by cytoplasmic protein concentration, but also determine cytoplasmic protein concentration. They found that protein synthesis was, in fact, maximal in speed at about 1x cytoplasmic concentration in line with Dill's conjecture. However, protein degradation was not; its speed continued to increase with cytoplasmic concentration well above 1x. This turns out to be a good thing; it means that if the concentration of proteins happens to drift above the nominal 1x level, the rate of synthesis will go down and the rate of degradation will go up, driving the system back toward 1x.
And why are the optima different for synthesis vs. degradation? It turns out that protein synthesis is more sensitive to increases in viscosity than degradation is.
Check it out here. And congrats to all!

Mar 2024: We are very pleased to announce the publication of a new paper from our own William Huang and our collaborator Steve Boxer. Here William looked at a very well known, but until now never really answered question: why does membrane translocation facilitate signal transduction? One highly plausible idea is that when you move a bunch of protein molecules from the cytoplasm to the plasma membrane, you decrease the amount of time needed for them to find their membrane-bound targets, in part because they are closer to their targets and in part because a 2D search is more efficient than a 3D search. The problem with this idea though is that proteins move much more slowly when they are bound to the plasma membrane than they do when they are in the cytoplasm, and as a result, several careful and smart theoreticians have argued that proteins should collide with their targets less frequently, not more frequently, when they are at the membrane. This would mean that some other advantage of being membrane-bound must be promoting signaling. Hmm....
William settled the issue (finally!) by carrying out a beautiful series of experiments. He measured the rate of an association reaction when the associating species (DNA strands) were in 3D versus when they were tethered to a 2D membrane. The answer: for a cell the size of a standard mammalian cell, association is 22- to 33-fold faster in 2D than in 3D. Analysis of the binding data shows that about half of the advantage comes from a faster collision rate in 2D, and about half from an increased probability of successfully binding after they collide.
It's just out in PNAS--have a look for yourself!
And, in other William Huang-related news, William has just begun an Asst. Professor position in the Dept. of Biophysics at Johns Hopkins University. We all wish him the very best!
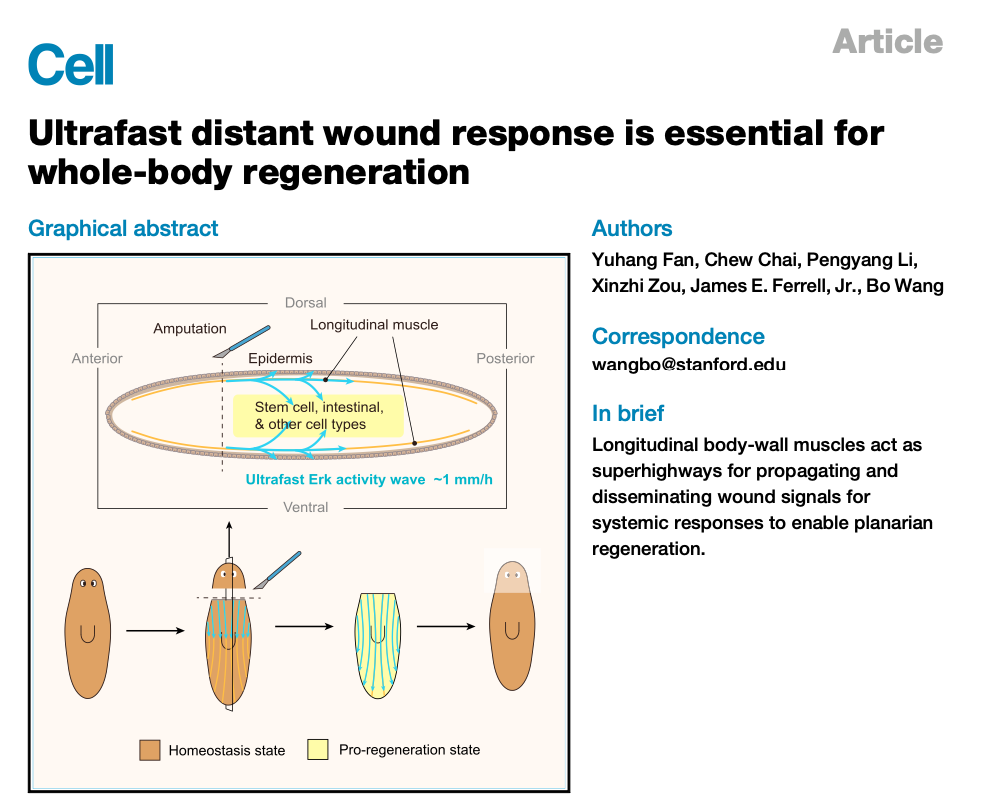
Aug 2023: Please check out the new paper from Yuhang Fan and several of his colleagues in Bo Wang's lab on regeneration in the planarian, now available online in Cell!
You may recall from 6th grade science class that if you cut a planarian in half tangentially or longitudinally, the worm fragments miraculously regenerate into normal looking, fully functional worms. Here Yuhang shows that when you cut a planarian, a regeneration signal spreads from the cut site to the furthest cells in each fragment, at a constant speed of ~5 mm per 5 h. This is way too fast to be due to diffusion of some messenger from the cut site, which would be expected to take ~350 h [since t = d2/2D = (5000 µm)2/(~20 µm2/s)]. It turns out that the mechanism is an intercellular trigger wave of Erk activation that propagates along tracts of longitudinal muscle cells. We think one 100 µm muscle cell globally activates its Erk in a process that takes about 5 min, and then releases something that starts another round of Erk activation in the next cell, and on and on, so that every 5 min the regeneration signal advances about 100 µm. This yet another example of how nature makes use of this cool trigger wave phenomenon to carry out long-distance communication.
Sep 2022: Our own William Huang and Ferrell lab alum Xianrui Cheng (and yours truly) have a new paper out in Nature Communications titled "Cytoplasmic organization promotes protein diffusion in Xenopus extracts". This work takes advantage of the fact that interphase Xenopus egg extracts are initially highly disorganized, and then, over the course of 30 min or so, miraculously self-organize into cell-like units with nicely organized organelles (see this paper for more details on extract self-organization, including video). For fluorescent probes that are the size of typical proteins (fluorescent BSA or 70 kDa dextran), self-organization is accompanied by a speeding of diffusion by about a factor of 2. This allows the probes to diffuse almost as quickly as they do in organelle-free cytosol. This phenomenon seems to be one way that cells generally speed up diffusion-controlled processes so that they don't have to live life at the torpid pace you might expect from their being so crammed full of diffusion barriers. Yay William and Xianrui!

Oct 2021: Jim's textbook is out. You can check it out here. The book presents some of the cool phenomena that emerge in cell signaling systems--ultrasensitivity, bistability, adaptation, oscillations, and excitability--together with the theory that allows you to understand why the systems behave the way they do. And it illustrates these phenomena and this theory through real life examples pulled from the systems biology literature. If you are a biologist who wants to learn about the dynamical systems that underpin developmental switches, heartbeats, and cell cycle oscillations, or a physicist who wants to learn how this non-linear dynamics stuff applies to the world of biology, this could be the book for you.
Aug 2021: Big congratulations to Yuping Chen, whose paper on C. elegans colony formation as a phase separation phenomenon is out now in Nat Commun. You can see the paper here.
Yuping had noticed that densely-plated Bristol N2 worms gather into round colonies when they run out of food. He characterized their movement, and showed that they run and then tumble every ~10 s or so, and that over time scales of minutes they are basically doing unbiased random walks. This allowed us to derive a simple formula showing why colonies only form when worms are present above a critical density, and showing that the density is determined by how much the worms slow down when they join a colony. Even though worms are millimeter-scale animals with brains, behavior, and the ability to crawl around via metabolic energy and muscles, this colony formation is formally analogous to phase separation phenomena exhibited by inanimate microscopic molecules being buffeted randomly by thermal motions—phenomena including micelle formation, precipitation of poorly-soluble solids, and the condensation of water vapor into liquid droplets.
Check it out!
Jan 2021: HAPPY NEW YEAR! Please join me in congratulating our own Julia Kamenz, who now has her own lab group at U Groningen! And also has a new paper in Current Biology on the amazing biphasic, bistable regulation of PP2A-B55, which you can see here. This work was a collaboration with our old friend Lendert Gelens, who now has his own lab at KU Leuven.
And please join me in congratulating Xianrui Cheng, who now has his own lab at the University of Sourthern California! All the best, Xianrui!
And, finally, please check out the new eLife paper by Oshri Afanzar! In collaboration with Garrison Buss and Tim Stearns, Oshri showed that the nucleus acts as the pacemaker for mitosis in Xenopus extracts. You can see it for yourself here.
Mar 2020: Want to understand how epidemiologists model diseases like COVID-19? Here is an easy-to-digest tutorial on the SIR (susceptible-infected-recovered) model and its application to the current pandemic (click here). And here is Mathematica code for the model (click here). We really recommend that you work through it--it's not that hard to do, and it really does allow one to think more clearly about this world-changing time we're all immersed in.
The bottom line of the modeling is that a disease as contagious as COVID-19 would be expected to infect 80% of the population. That's where you end up once you have depleted the pool of "susceptibles" enough to make the epidemic fizzle out. But, and this is a big but, this percentage can, in principle, be reduced to a tiny fraction of a percent if we reduce the number of secondary infection per primary infection below the critical value of 1. SO PLEASE TAKE SOCIAL DISTANCING SERIOUSLY!
Nov 2019: What happens if you put a computer in a blender and homogenize it for a while? You'll get little bits of ground up computer. And those little bits just sit there and can't do much of anything. Not even trigonometry.
So what happens if you take frog eggs and homogenize them into cytoplasmic extract? Well, they start out as a random-looking mess, but then over the next 30 min they turn into what looks like a field of cells. And if you let the extract synthesize proteins, these cell-like structures divide, and divide again, and so on. Who knew that the living state had such an ability to put itself back together?
Xianrui Cheng just published a cool paper on this in Science. Check it out!
May 2019: And one more new paper, from Tony Tsai and his collaborators in Julie Theriot's lab and Tobias Meyer's lab. For those of you who don't know, Tony completed a Ph.D. thesis project in the Ferrell lab and one in the Theriot lab (kids, don't try this at home), and his Theriot lab work, on neutrophil migration, is now out in Dev Cell. Congrats Tony!
May 2019: A couple of new papers from our collaborators in Michael Lin's and Steve Artandi's labs have just been published. The first is from grad student Kay Chung and Michael on their invention and development of what they call RASER (for Rewiring of Aberrant Signaling to Effector Release), a synthetic protein circuit that selectively kills cells whose EGF receptors are chronically activated. It's a great idea and, moreover, they were able to take it from great idea to successful implementation through a lot of hard work and a little ODE modeling. The work appeared in the May 3 issue of Science.
The second is from grad student Caitlin Roake and Steve on the processing of telomerase RNA. ODE modeling helped here too--to make sense of some experimental data on an RNA maturation pathway that is too complicated to understand without writing down explicit reaction steps and simulating them. Caitlin's paper was published in Mol Cell on Mar 29.
Congratulations to Caitlin and Steve, and to Michael and Kay, on their beautiful work!
May 2019: Welcome to new postdoctoral fellows William Huang, from Jay Groves's lab at UC Berkeley, and Yuping Chen from Bruce Futcher's lab at Stony Brook U. William is working on single molecule studies of signaling in reconstituted Xenopus extract/membrane systems, and Yuping is working on the interactions between waves of Cdk activity in Xenopus extracts. Once more unto the breach, dear friends!
 Aug 2018: Our own Xianrui Cheng has a paper in the Aug 10 2018 issue of Science on apoptotic trigger waves, which shows, as you might guess, that apoptosis spreads through the cytoplasm via trigger waves. Congratulations Dr. Cheng! You can get to the paper by clicking here, and if that's not enough for you, check out the articles about the article in Cosmos, The Guardian, Newsweek, Science News, New Scientist, Stanford Medicine, and/or Digital Trends, or listen to the Aug 16 podcast of Science in Action on the BBC World Service.
Aug 2018: Our own Xianrui Cheng has a paper in the Aug 10 2018 issue of Science on apoptotic trigger waves, which shows, as you might guess, that apoptosis spreads through the cytoplasm via trigger waves. Congratulations Dr. Cheng! You can get to the paper by clicking here, and if that's not enough for you, check out the articles about the article in Cosmos, The Guardian, Newsweek, Science News, New Scientist, Stanford Medicine, and/or Digital Trends, or listen to the Aug 16 podcast of Science in Action on the BBC World Service.
Aug 2018: A fond farewell to our summer crew, Salvador Buse, Annie Carilli, and Alexandra Vandervelde—visiting undergrad, undergrad, and postdoc, repectively. It has been just great having you all with us. All the best for the school year, and come back soon!
Jul 2018: And a warm welcome to Dr. Oshri Afanzar, who comes to us from the Weizmann Institute as a postdoctoral fellow. Oshri was a Ph.D. student with Michael Eisenbach, working on the regulation of flagellar rotation in E. coli. Oshri will be working on trigger waves, building upon the nice work that Jeremy Chang and Xianrui Cheng have done. It's great to have you here, Oshri!
Mar 2018: Hello prospective Stanford Bioscience Ph.D. students! We look forward to getting to know you over the next couple of days.
Jan 2018: A warm welcome to Dr. Yuxin Chen, who joined the lab as a postdoctoral fellow. Yuxin was a Ph.D. student with Chung-I Wu at Sun Yat-Sen University in Guangzhou China, working on the evolution and stability of gene regulatory networks. He's now focusing ona project on the spatial spread of innate immune responses. Welcome Yuxin!
And to Lawrence Chiou, a Biophysics Ph.D. student who has now joined the lab. Lawrence is working on temperature scaling, and is also looking at a mesoscopic example of something that is much like the liquid-liquid phase transitions that are cropping up in various biological contexts. Welcome Lawrence!
Oct 2017: The Oct 3 2017 issue of Cell Reports features an article from Graham Anderson and Lendert Gelens on the waves of cell division that pass through the Xenopus embryo during early development. The cover picture shows a dividing embryo that was placed between two mirrors tilted at 45° angles to allow the embryo to be simultaneously filmed from the top and theside (two sides, actually). The work was a collaboration between the Ferrell lab and Julie Baker's lab in the Dept. of Genetics. Yay team!
Graham's and Lendert's work started with an old observation: when Xenopus embryos cleave, first in two, then in four, and so on, the cell divisions happen almost, but not quite, synchronously. If you look carefully you can see that there is actually a wave of cell division that sweeps from one side of the embryo to the other. Graham and Lendert set out to answer two questions: (1) how does the wave of cell divisions arise, and (2) is the near-synchrony of the embryonic divisions important for normal embryogenesis?
For question 1: They had two plausible working hypotheses. The first was that cell division happened in one cell first, and then spread (somehow) from cell to cell through the embryo. This is how a contraction wave spreads in the human heart. The second was that all of the cells in the embryo had their own independent cell cycle "clock", with some clocks pre-programmed to run faster and some to run slower. In this case the wave of cell divisions would be what is termed a kinematic wave, like the flashing lights on a movie marquee, with the apparent cell-to-cell spread being an illusion.
To distinguish between these possibilities, Graham and Lendert placed Xenopus embryos in a temperature gradient for an hour or so, yielding embryos where the cells on one side of the embryo were advanced in phase relative to the cells on the other side. The embryos were then restored to a constant temperature, and Graham and Lendert looked to see whether the desynchronized cells would come back into sync, as would be expected if cell cycles can spread from cell-to-cell. The resulty was that the cells never resynchronized. It seems that the cell division wave is a kinematic wave, an illusion due to differences in how fast the cell cycle clock runs in different parts of the embryo.
For question 2: One of the earliest differentiation events in embryogenesis is mesoderm induction. As early as the 32-64 cell stage, signals from the bottom (vegetal) half of the embryo cause cells just above the middle to turn into mesoderm, and then the mesoderm is patterned. It seemed plausible that if the cells doing the inducing/pattern and the cells receiving the inducing/patterning signals were out of sync with each other, mesoderm induction would be disrupted. And, indeed, Graham and Lendert found that it was. To our surprise, though, it turned out that this abnormal patterning was corrected later in embryogenesis, after the 4000-cell stage (the midblastula transition), so that the desynchronized embryos ultimately developed into normal tadpoles. This finding underscores the robustness of the overall program of embryogenesis.
Congratulations Graham and Lendert!
Sep 2017: It's time again for Stanford's own Loose Ends, with Jim on guitar, to make loud rock music while you all eat free burgers and drink free beer on the Clark Center's Nexus Cafe Patio. The happenings will be Friday Sep 29 from 4-6 PM—be there or be hungry, sober, and less deaf.
 Sep 2017: And now we must bid a fond farewell to Andreas Meyerhans. During his time with us he learned some systems biology and taught us some immunology. He also came up with a great idea for a new trigger wave project that we plan to pursue collaboratively. And he got the chance to hit those bongos at the CSB Retreat this month, jamming with the Mighty Temblors. So long, Andreas!
Sep 2017: And now we must bid a fond farewell to Andreas Meyerhans. During his time with us he learned some systems biology and taught us some immunology. He also came up with a great idea for a new trigger wave project that we plan to pursue collaboratively. And he got the chance to hit those bongos at the CSB Retreat this month, jamming with the Mighty Temblors. So long, Andreas!
Aug 2017: Upcoming talks—Jim will be speaking at the SystemsX Conference on Systems Biology in Zurich Sep 4; at the University of Amsterdam Sep 8; and at the Weizmann Institute in Rehovot Israel on Sep 11. If you are in the area, please stop by and say hello.
Jun 2017: Welcome Andreas Meyerhans! Andreas is spending the next four months with us as a sabbitical visitor. He hails from Barcelona, where he is an ICREA Research Professor at the University of Pompeu Fabra. His research focuses on the interactions between viruses and theire hosts in vivo, with a particular focus on what governs whether a viral infection will be acute but ultimately cleared, or chronic and persistent--questions that call out for some new insights from nonlinear dynamices. Welcome Andreas!
May 2017: And a fond farewell to Tek Hyung Lee, who is moving to a position at Lawrence Livermore National Laboratory. Tek Hyung will be working with Mimi Cho Yung, engineering bacteria for protein expression. All the best, Tek Hyung!
Apr 2017: And another bittersweet bye bye! We bid a fond farewell to Graham Anderson, who has joined Ferrell lab alum Zach Serber's company, Zymergen. Congratulations, Dr. Anderson--an era ends and another begins.
Feb 2017: Bye bye! We bid a fond farewell to Sang Hoon Ha, who is moving on to take a faculty position at Chonbuk National University in Korea. Congratulations on the new job, Sang Hoon, and all the best from all of us.
Feb 2017: Welcome Minjung Kang! Min was a Ph.D. student at Weill Cornell Medical School, working with Kat Hadjantonakis, and has now arrived in rainy California to do her postdoctoral work in the Ferrell lab. She is working on mitotic regulation in mammalian cells.
Feb 2017: Julia Kamenz and Jim have written a commentary on the temporal ordering of cell cycle phosphorylation, which was published Feb. 2 in Molecular Cell. Julia has also been selected to give a talk on her work on this topic at the Winter qBio Meeting in Kauai Feb 24-27.
Jan 2017: Jim gave a talk on Xianrui Cheng's work on apoptosis at the Gordon Research Conference on Stochastic Physics in Biology in Ventura CA, Jan 8-13. The high point of the conference was a talk by Dan Gillespie on the skeptical response his now-famous algorithm initially received from the chemical physics community, and on his new work extending the algorithm to systems where the reactants occupy non-negligible volumes (like living systems).
Dec 2016: Welcome, Connie Phong! Connie was a Ph.D. student in Mike Rust's lab at the University of Chicago, and has now joined us as a postdoctoral fellow. Connie will be working on temperature scaling in cell cycle regulation in collaboration with our old friend Lendert Gelens.
Oct 2016: Congratulations to Sarah Trosin, who successfully defended her Ph.D. thesis October 19. The title of her presentation was "Toward Understanding Cyclin Requirements for Mitotic Entry in the Mammalian Somatic Cell Cycle".
Oct 1 2016: Congratulations to our own Xianrui Cheng, who tied for the silver medal for best talk at the CSB Annual Retreat at Stanford Sierra Camp. And congratulations to the other medalists, Stephan Hamperl (form Karlene Cimprich's lab) and Opher Kornfeld (from Daria Mochly-Rosen's lab). And to everyone else too--the quality of the research and the talks was really extraordinary this year!
July 19 2016: Jim lectured at the 10th Annual q-Bio Summer School at Colorado State.
 July 10 2016: The Loose Ends, a Stanford rock band with Jim on guitar, will be playing at the Clark Center this Friday, July 15, from 4:00-6:00 on the Nexus Cafe Patio. In addition to that loud rock music the kids all dig, there will be free food and beer.
July 10 2016: The Loose Ends, a Stanford rock band with Jim on guitar, will be playing at the Clark Center this Friday, July 15, from 4:00-6:00 on the Nexus Cafe Patio. In addition to that loud rock music the kids all dig, there will be free food and beer.
July 4 2016: Cell Systems featured the Ha and Ferrell Science paper in the latest in their "Principles of Systems Biology" series (#6). A link to the feature can be found here. The paper was also recently recommended by the Faculty of 1000.
June 13 2016: Jim will be speaking this Thursday at the International Conference on Systems Biology of Human Disease (SBHD), at the Broad Institute in Cambridge.
June 6 2016: Congratulations to Julia Kamenz, who has been awarded a Deutsche Forschungsgemeinschaft (DFG) Postdoctoral Fellowship to support her work on the ordering of mitotic events!
May 25 2016: The latest issue of Science Signaling highlighted the recent Ha and Ferrell Science article as a "Paper of Note" (click here).
May 19 2016: Our own Julia Kamenz has been selected as a speaker for this year's Cold Spring Harbor Cell Cycle meeting. Julia will speak this Saturday (May 21) about her work on protein dephosphorylation during mitotic exit.
May 13 2016: We have a new paper out on thresholds and negative cooperativity. Here's a quick summary:
In the 1960's, Dan Koshland introduced the idea of "negative cooperativity" in the function of proteins with multiple subunits. In negative cooperativity, the binding of one ligand makes it harder for a second ligand to bind. The standard theory on negative cooperativity says that the higher the negative cooperativity, the more graded the receptor's response.
It turns out there is a little algebraic shortcut built into the standard theory--effectively it is an assumption that the binding of ligand molecules to receptors has no significant impact on the concentration of free ligand. In many situations, especially intracellular signaling, this assumption does not hold.
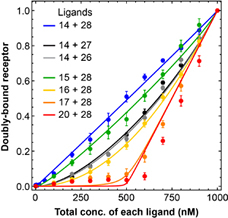 If one re-derives the theory without this little shortcut, the results are different, and kind of remarkable. Negative cooperativity can endow a receptor's response with a marked threshold, making it so there will be no response until the ligand concentration is higher than the receptor concentration. Sang Hoon Ha tested the new theory with a series of synthetic biology experiments, using DNA oligonucleotides to represent the receptor and ligand species and manipulating the cooperativity of their binding. The results are in beautiful agreement with the new theory.
If one re-derives the theory without this little shortcut, the results are different, and kind of remarkable. Negative cooperativity can endow a receptor's response with a marked threshold, making it so there will be no response until the ligand concentration is higher than the receptor concentration. Sang Hoon Ha tested the new theory with a series of synthetic biology experiments, using DNA oligonucleotides to represent the receptor and ligand species and manipulating the cooperativity of their binding. The results are in beautiful agreement with the new theory.
The paper has just been published in Science (click here). Congratulations Sang Hoon!
 Apr 29 2016: Jim is just back from the University of Texas Southwestern, where he presented the 16th annual Rupert E. Billingham Lecture in Cell Biology. Jim talked about trigger waves in intracellular communication.
Apr 29 2016: Jim is just back from the University of Texas Southwestern, where he presented the 16th annual Rupert E. Billingham Lecture in Cell Biology. Jim talked about trigger waves in intracellular communication.
Mar 2016: Bye bye! As of April 1, our own Lendert Gelens leaves Stanford Chemical and Systems Biology and begins as an Asst. Professor at the University of Leuven in Belgium. He will head the Dynamics in Biological Systems (DiBS) lab. We are all looking forward to watching this exceptionally able young scientist become one of the leading lights in quantitative biology. Good luck, Lendert!
Feb 2016: Jim speaks at the Biophysical Society's 60th Annual Meeting in the Synthetic Biology Symposium, which is Sun Feb 28 from 8:15-10:15 AM. So does Stanford Chemical and Systems Biology's own Mary Teruel. More info? Click here.
Feb 2016: Jim's review on perfect adaptation was just published in Cell Systems. The article disusses the Holy Trinity of adaptation mechanisms—negative feedback, incoherent feedforward regulation, and state-dependent inactivation—as well as a new twist on negative feedback recently proposed by Mustafa Khammash and his colleagues (click here). As important as at is to understand how signaling systems are turned on, it is nice to be getting a comprehensive picture of what nature does to ensure that they get turned back off.
Feb 2016: We are just back from the Winter q-Bio meeting in Honolulu. Lendert Gelens gave a talk about his and Graham Anderson's unpublished work on waves of mitosis in embryos. Jim presented Xianrui Cheng's unpublished work on apoptotic trigger waves (Hawaii is a good place for waves!) and Sanghoon Ha's unpublished work on negative cooperativity. Ferrell lab alumna Silvia Santos also spoke, presenting new stuff on the temporal modularity of mitosis in mammalian cells. A splendid time was had by all!
 Feb 2016: Please check out the latest paper from Sanghoon Ha and Sun Young Kim, just published in Cell Reports. The paper takes on the question of why it is that the Suc1/Cks proteins, components of the cyclin B-Cdk1 complex, seem to both promote and inhibit cell cycle progression. The answer lies in the prozone effect, where adaptors like Cks proteins can promote complex formation at substoichiometric concentrations, but then inhibit complex formation at suprastoichiometric concentrations. The paper is a nice combination of theory, proof-of-principle experiments, and in vitro studies of cyclin B1-Cdk1-Cks2 biochemistry.
Feb 2016: Please check out the latest paper from Sanghoon Ha and Sun Young Kim, just published in Cell Reports. The paper takes on the question of why it is that the Suc1/Cks proteins, components of the cyclin B-Cdk1 complex, seem to both promote and inhibit cell cycle progression. The answer lies in the prozone effect, where adaptors like Cks proteins can promote complex formation at substoichiometric concentrations, but then inhibit complex formation at suprastoichiometric concentrations. The paper is a nice combination of theory, proof-of-principle experiments, and in vitro studies of cyclin B1-Cdk1-Cks2 biochemistry.
Nov 2015: The EMBL Symposium on "Biological Oscillators: Design, Mechanism, and Function" took place Nov 12-14 at EMBL in Heidelberg. Jim spoke about how the reactions underpinning a biological oscillator can determine the spatial organization of the oscillations.
Oct 2015: Congratulations to Graham Anderson, who successfully defended his Ph.D. thesis, in fine style, on Oct 30. The title of his presentation was "Embryonic Division Waves Develop Autonomously and Precede Proper Mesoderm Induction".
Oct 2015: Congratulations to Dr. Lendert Gelens, whose talk at the "Physics of Living Matter" symposium in Cambridge UK was awarded the "Best Young Researcher Prize". Dr. Gelens spoke on the spatial coordination of development in Xenopus embryos subject to temperature perturbations. The work was done in collaboration with Graham Anderson from the Ferrell lab and K. C. Huang from the Dept. of Bioengineering.



