Collman Group
20+ years of excellence in metalloporphyrin chemistry
|
|
|
|
|
|
Biomimetic studies of Nitric Oxide Reductase
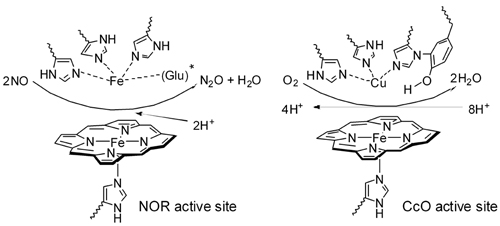
· Nitric oxide reductase (NOR) is a membrane-bound enzyme which catalyzes the 2e- reduction of nitric oxide to nitrous oxide, an obligatory step involved in the sequential reduction of nitrate to dinitrogen known as bacterial denitrification. The active site of NOR consists of a monohistidine ligated five-coordinate heme and a trisimidazole ligated non-heme FeB. This structure strongly resembles the active site of oxygen reduction enzyme-cytochrome c oxidase (CcO) which possesses a heme-a3/CuB center (Figure 1). Essentially, the distal metal CuB in CcO is replaced by a non-heme Fe metal in NOR; NOR and CcO are thought to be distant relatives.
Figure 1
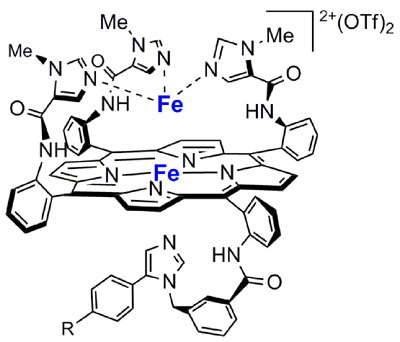
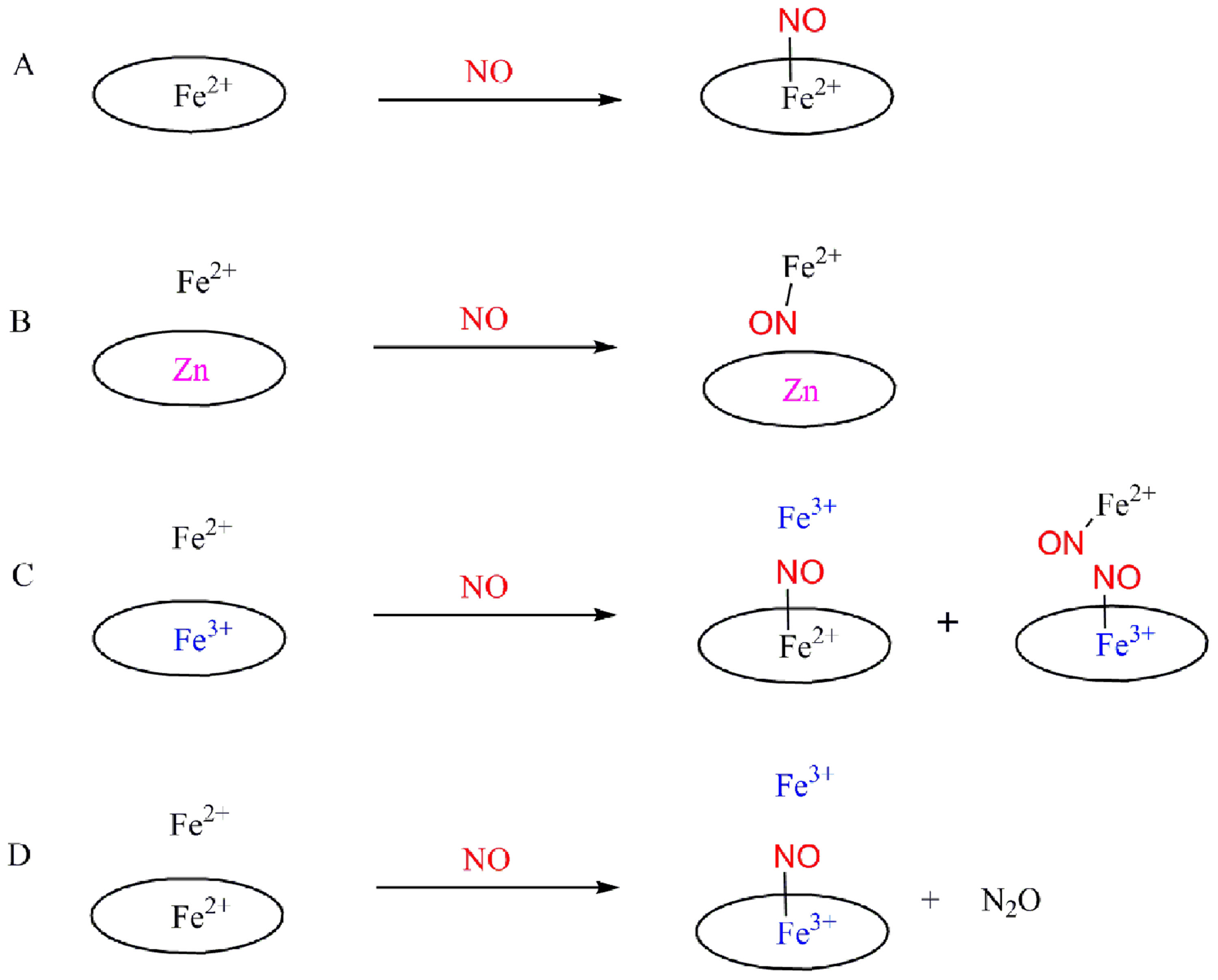
· The first functional heme/non-heme nitric oxide reductase (NOR) model (Figure 2) is presented. The fully reduced diiron compound reacts with two equivalents of NO leading to the formation of one equivalent of N2O and the bis-ferric product. NO binds to both heme Fe and non-heme Fe complexes forming individual ferrous nitrosyl species. The mixed-valence species with an oxidized heme and a reduced non-heme FeB does not show NO reduction activity (Scheme 1). These results are consistent with a so-called "trans" mechanism for the reduction of NO by NOR .
Figure 2
Scheme 1